Fig. 15.1
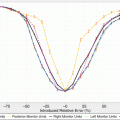
(a) The average chart for the clinical case. Subgroup size of four is used to verify the constancy of the flatness and symmetry. (b) The range chart of the four periphery ion chambers in the RBA-5 device (Reprint from Pawlicki et al. [12])
The reason why a descriptive statistic, such as the standard deviation, is ineffective as a method to set action thresholds for quality assurance is that it tends to obscure the temporal structure of the data. It should be emphasized that clinical requirements will always have precedence over any method of setting action thresholds. If the action thresholds on the process behavior charts are outside the clinical requirements, then the only course of action is to reengineer the process with better equipment and/or procedures.
As dynamic data are continuously generated from a QA process, it is often difficult to identify when a single point displays highly nonrandom behavior and requires further investigation. Process behavior charts separate variation into two sources: variation due to systematic sources for which there is an assignable cause and variation due to random sources for which there is no readily assignable cause. One should search for an assignable cause when a data point exceeds an action threshold on the process behavior chart. After an assignable cause is found, measures can then be incorporated to reduce or eliminate it from the process. When the process is subject to only random variation, the process is predictable, and the limits on the process behavior chart describe the process potential.
15.3 Geometric Verification of Treatment Delivery
Stereotactic body radiotherapy (SBRT) is being increasingly employed as an alternative modality for the treatment of primary and secondary cancers [17, 18]. SBRT has the important advantages of shortened treatment times while delivering higher biologically effective doses. However, normal tissues surrounding the tumors are also exposed to high-dose levels of radiation. Furthermore, cancerous tissue can occasionally move outside the irradiation field, e.g., when the patient has irregular breathing or episodes of coughing. Under these circumstances, malignant tissue will be missed, and more normal tissue than planned will be irradiated. Consequently, the precision requirement of SBRT is high. It is absolutely critical to effectively monitor the target to ensure maximal irradiation of the tumor with minimal irradiation of surrounding normal tissue [8].
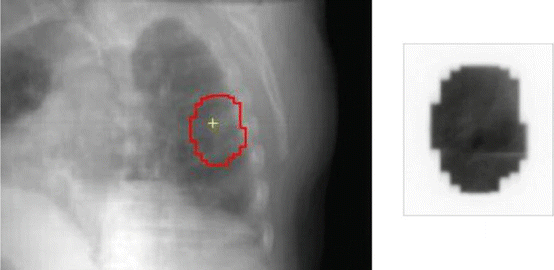
Compared with on-board kV imaging, EPID (electronic portal imaging device) acquisition in the cine mode provides a number of advantages for treatment verification purposes: (1) it utilizes the MV treatment beam for imaging and does not involve any additional radiation dose to the patient, and (2) it shows what is actually being irradiated by giving the beam-eye-view of the patient anatomy. Berbeco et al. [1, 2] developed a matching technique for respiratory-gated liver radiotherapy treatment verification with an EPID in the cine mode. Implanted radiopaque fiducial markers inside or near the target were required for this technique. Markerless techniques were also proposed [13, 14]. However, due to the degraded quality of MV images, it is often very challenging to visualize the tumor target in cine EPID images.
Tang et al. [16] recently proposed a novel approach for SBRT treatment verification using cine EPID images based on a machine learning algorithm. They modeled the treatment verification problem as a two-class classification problem and applied an artificial neural network (ANN) to classify the cine EPID images acquired during the treatment into corresponding classes—with the tumor inside or outside of the beam aperture. Training samples were generated for the ANN using digitally reconstructed radiographs (DRRs) with artificially added shifts in the tumor location—to simulate cine EPID images with different tumor locations (Fig. 15.2). Principal component analysis (PCA) was used to reduce the dimensionality of the training samples and cine EPID images acquired during the treatment. The proposed treatment verification algorithm was tested on five lung SBRT patients in a retrospective fashion. On average, the machine learning algorithm achieved very high classification accuracy, recall rate, and precision rate, all in the high 90 %. For its practical implementation, a comprehensive clinical validation remains to be performed in terms of different tumor volumes, location, and imaging angle. In addition, the algorithm’s performance for cine MV images acquired with partial field of view due to modulated treatments such as IMRT or VMAT needs to be evaluated.
15.4 Dosimetric Verification of Treatment Delivery
Patient-specific measurements are typically used to validate the dosimetry of complex treatments such as IMRT and VMAT. To evaluate the dosimetric performance over time of the treatment delivery process, Breen et al. [3] used statistical process control (SPC) concepts to analyze the measurements from 330 head-and-neck (H&N) treatment plans. H&N IMRT cases were planned with the PINNACLE3 treatment planning system (Philips Medical Systems, Madison, WI) and treated on Varian (Palo Alto, CA) or Elekta (Crawley, UK) linacs. As part of regular quality assurance, plans were recalculated on a 20-cm-diameter cylindrical phantom, and ion chamber measurements were made in high-dose volumes (the PTV with highest dose) and in low-dose volumes (spinal cord organ-at-risk, OR). Differences between the planned and measured doses were recorded as a percentage of the planned dose. It was demonstrated that head-and-neck IMRT plans could be delivered with a systematic error of 0.2 % in high-dose volumes and −1.0 % in low-dose volumes.
Statistical process control also provides a means to evaluate adjustments to the process (via beam models) in a robust manner. For IMRT dosimetric verification, measurements in phantom are not the end of the QA process; rather, they are the data upon which a strong foundation of continuous quality improvement can be constructed. Analysis of this large series of H&N IMRT measurements demonstrated that the IMRT dosimetry was stable over time and within accepted tolerances. These data provide useful information for assessing alterations to beam models in the planning system. IMRT is enhanced by the addition of statistical process control to traditional quality control procedures.
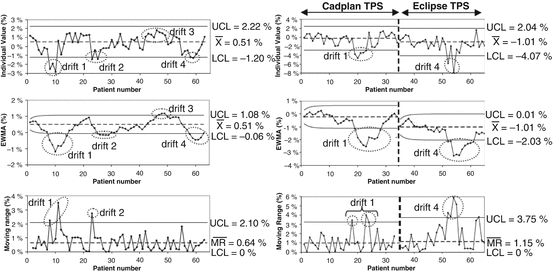
Gerard et al. [7] further investigated the use of two complementary machine learning tools—control charts and performance indices—to accurately analyze the dose delivery process in IMRT. Control charts aim at monitoring the process over time using statistical control limits, whereas performance indices aim at quantifying the ability of the process to produce data that are within the clinical specification limits at a precise moment. They showed that three control charts—individual value, moving range, and exponentially weighted moving average (EWMA) control charts, chosen for their capacity of bringing complementary information—allowed an efficient detection of the drifts that occurred in the IMRT dose delivery process for prostate and head-and-neck treatments (see Fig. 15.3). The dose delivery process for prostate treatments was both statistically in control and capable, i.e., its evolution can be predicted within the clinical specification limits. For head-and-neck treatments, the dose delivery process was in control but not statistically capable, when using the current specification limits set at 4 %. This implies that the evolution of the process can be predicted but not within the specification limits. So, as shown by the process performance indices, actions should be undertaken to improve both the process centering and dispersion.