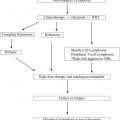
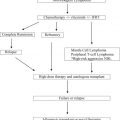
Myelodysplastic syndromes are one of the most common hematological disorders in the elderly. Therefore, an increase in the prevalence of de novo but also of secondary forms after prior chemotherapy or radiotherapy, respectively, is anticipated within the next years. Allogeneic stem cell transplantation is considered the only potentially curable therapy, but many patients are not eligible because of age or comorbidities. Reduced-intensity conditioning regimens have improved early tolerability of the procedure, although late effects remain a challenge in the care of these patients. However, hypomethylating agents have become available as alternative therapeutic approaches with a moderate toxicity profile.
Key points
- •
Transplantation can be a potential curative option in elderly patients with myelodysplastic syndromes within prospective trials investigating the success of allogeneic stem cell transplantation (SCT) compared with other treatment options.
- •
In the absence of prospective trials, a careful individual selection should be done and patients should be stratified according to comorbidities, performance status, and disease risk.
- •
Chronic graft-versus-host disease (GVHD) and relapse are still the major challenges after SCT.
- •
Special attention should be paid to posttransplant care in terms of GVHD management, minimal residual disease (MRD) monitoring, and prevention of relapse.
Introduction
Because of the great variability of biology and presentation of the disease, the therapeutic management of patients with myelodysplastic syndromes (MDS) is challenging. Over the last decade, there has been a remarkable increase in scientific research on the pathogenesis and therapeutic approaches in MDS. However, despite a rapidly expanding therapeutic drug arsenal, allogeneic hematopoietic stem cell transplantation (SCT) remains the only potentially curative treatment. Only a minority of patients are considered for this treatment modality because cumulative mortality rates after SCT can exceed 30% and also because graft-versus-host disease (GVHD) as well as relapse remain major clinical challenges following transplantation. Because of the heterogeneous nature of MDS, ranging from low-risk patients with a life expectancy of 10 years with best supportive care only to high-risk patients with MDS with a median survival of 5 months with the very best standard treatment, not every patient with MDS will benefit from this potential curative but risky treatment procedure. In fact, physicians who treat patients with MDS are faced primarily with an older patient population (median age of 70 years at diagnosis) with significant comorbidities and reduced biological ability to regenerate. In these patients, the potential high treatment-related mortality (TRM) of standard conditioning would limit the benefit of this procedure. With the introduction of reduced-intensity conditioning (RIC), early TRM could be reduced, allowing a potentially curative treatment approach even for patients in the seventh decade of life. On the other hand, the use of RIC is accompanied by a higher risk of relapse, especially in patients with higher-risk disease. Defining patients who should be referred to allogeneic SCT and the best time point within the treatment course of MDS is, therefore, essential. In fact, prospective randomized trials supporting these decisions are still not available. This review highlights the current selection process and therapeutic strategies before and after allogeneic SCT to potentially improve the outcome of patients with MDS undergoing this procedure.
Introduction
Because of the great variability of biology and presentation of the disease, the therapeutic management of patients with myelodysplastic syndromes (MDS) is challenging. Over the last decade, there has been a remarkable increase in scientific research on the pathogenesis and therapeutic approaches in MDS. However, despite a rapidly expanding therapeutic drug arsenal, allogeneic hematopoietic stem cell transplantation (SCT) remains the only potentially curative treatment. Only a minority of patients are considered for this treatment modality because cumulative mortality rates after SCT can exceed 30% and also because graft-versus-host disease (GVHD) as well as relapse remain major clinical challenges following transplantation. Because of the heterogeneous nature of MDS, ranging from low-risk patients with a life expectancy of 10 years with best supportive care only to high-risk patients with MDS with a median survival of 5 months with the very best standard treatment, not every patient with MDS will benefit from this potential curative but risky treatment procedure. In fact, physicians who treat patients with MDS are faced primarily with an older patient population (median age of 70 years at diagnosis) with significant comorbidities and reduced biological ability to regenerate. In these patients, the potential high treatment-related mortality (TRM) of standard conditioning would limit the benefit of this procedure. With the introduction of reduced-intensity conditioning (RIC), early TRM could be reduced, allowing a potentially curative treatment approach even for patients in the seventh decade of life. On the other hand, the use of RIC is accompanied by a higher risk of relapse, especially in patients with higher-risk disease. Defining patients who should be referred to allogeneic SCT and the best time point within the treatment course of MDS is, therefore, essential. In fact, prospective randomized trials supporting these decisions are still not available. This review highlights the current selection process and therapeutic strategies before and after allogeneic SCT to potentially improve the outcome of patients with MDS undergoing this procedure.
Patient evaluation overview
Diagnostic and Prognosis
According to the recently published guidelines from the European LeukemiaNet (ELN) ( Table 1 ), every patient should receive a complete blood count, a peripheral blood (PB) smear with differential leukocyte count, a bone marrow (BM) aspiration for morphologic evaluation, as well as a BM biopsy to assess marrow fibrosis. Furthermore, cytogenetic analysis of BM should be undertaken in all cases of suspected MDS. At least 20 metaphases have to be analyzed; if less than 20 metaphases are scored, then the results should be considered invalid, provided the karyotype appears normal. Fluorescence in situ hybridization analysis, with a validated panel of probes, is recommended in certain instances (eg, to clarify complex aberrations, to detect specific anomalies [eg, monosomy 7 or del (5q)], or when only a low number of metaphases are available for standard G banding).
| Diagnostic Tool | Diagnostic Value | |
|---|---|---|
| 1. Peripheral blood smear | Evaluation of dysplasia and blast count | Mandatory |
| 2. Bone marrow aspirate | Evaluation of dysplasia and blast count, Quantification of ring sideroblasts | |
| 3. Bone marrow biopsy | Assessment of cellularity, CD34+ count and fibrosis | |
| 4. Cytogenetic analysis | Detection of clonal chromosomal abnormalities for conclusive diagnosis/prognostic assessment | |
| 5. FISH | Detection of targeted chromosomal abnormalities in interphase nuclei following repeated failure of standard G banding | Recommended |
| 6. Immunophenotyping | Detection of abnormalities in erythroid, myeloid, and lymphoid compartment | |
| 7. SNP-array | Detection of chromosomal defects at a high resolution in combination with metaphase cytogenetic | Suggested |
| 8. Mutation analysis of candidates genes | Detection of somatic mutations that can allow a conclusive diagnosis and also reliable for prognostic evaluation |
The cytomorphological assessment of the bone marrow smear is the basic diagnostic when an MDS is suspected. As a result of cytogenetic and epigenetic changes, there will be found more or less pronounced morphologic alterations in at least 1 cell line up to trilinear dysplasia and is associated with a normal or increased blast count.
Many clinical trials use the IPSS for differentiation between lower- versus higher-risk disease. However, some patients classified as lower risk, particularly those in the IPSS intermediate-1 group, may have higher-risk features (eg, patients with red blood cell [RBC] transfusion–dependent [TD] refractory cytopenia with multilineage dysplasia [RCMD] with intermediate karyotype). Consequently, a revised IPSS (IPSS-R) has been proposed, which incorporates several changes, including an updated system for assessing cytogenetic risk and the consideration of the severity of cytopenias (with thresholds of hemoglobin: 10 g/dL; absolute neutrophil count: 0.8 × 10 9 L −1 ; platelets: 100 × 10 9 L −1 ). Using this new R-IPSS, 27% of lower-risk patients with MDS per classic IPSS were reclassified as having a higher risk and may potentially require more intensive treatment, whereas 18% of higher-risk patients per classic IPSS were reclassified as low risk, suggesting that IPSS-R can refine the classification of MDS and more individual prognosis estimation is possible. Still, the benefit of IPSS-R for guided therapy decisions is under investigation. Quite recently, Della Porta and colleagues developed an IPSS-R–based score to predict outcome of patients after SCT.
Chromosomal aberrations are present in approximately 50% of patients with MDS. The impact of cytogenetic risk groups on prognosis and the rate of transformation to acute myeloid leukemia (AML) are well known. Moreover, the selection of certain therapeutic options may be driven by the existence of specific chromosomal abnormalities. For example, lenalidomide (Revlimid) is particularly effective in patients with low-risk, TD MDS with a deletion of chromosome 5q.
More frequently, there are somatic mutations, which are, however, not exclusively found in MDS. Mutations (eg, in regulator genes of central pathways in the pathophysiology in MDS) are suitable as prognostic parameters and may also be, in the future, a specific therapeutic target. An extract of recurrent mutations in MDS is listed in Table 2 .
| Function | Mutation | Prognosis | Frequency (%) |
|---|---|---|---|
| Splicing | SF3B1 | Good | 15–30 |
| SRSF2 | Poor | 5–10 | |
| U2AF1 | Poor | 5–10 | |
| ZRSR2 | Neutral | 5 | |
| Methylation | DNMT3A | Poor | 5–10 |
| TET2 | Neutral | 15–25 | |
| Methylation/histone modifications | IDH1/IDH2 | Mixed evidence | 4–5 |
| Histone modification | ASXL1 | Poor | 10–20 |
| EZH2 | Poor | 3–7 | |
| Transcription factor | RUNX1 | Poor | 5–10 |
| TP53 | Poor | 5–10 | |
| BCOR-L1 | Poor | 5–6 | |
| ETV6 | Poor | 3 | |
| Signal transduction | NRAS/KRAS | Poor | 5–10 |
Accurate diagnostics and subsequent prognostication are crucial in determining the appropriate treatment, including best supportive care, immunomodulatory drugs, chemotherapy, or SCT. Additionally, accompanying diseases, preexisting organ dysfunction, clinical condition and symptoms, as well as the biological age must be considered carefully. The Seattle group developed a hematopoietic cell transplantation specific comorbidity index (CI) to predict the influence of the pretransplant comorbidities on TRM and overall survival (OS) in hematopoietic SCT recipients. In a Web-based available questionnaire ( www.HCTci.org ), hepatic, pulmonary, cardiac, and renal impairments as well as other pretransplant variables can be translated into a patient-specific score. The predictive value of this score on the posttransplant outcome has been evaluated in different studies worldwide. Furthermore, the predictive relevance of patients’ physical condition on outcomes has been shown by Deschler and colleagues with several performance status scores being predictive for post-SCT mortality.
Indications for transplantation
First, we have to be aware of the fact that allogeneic SCT is the only potentially curative therapy for MDS; but there is finally no warranty for treatment success. As pointed out earlier, individual risk assessment determined by disease-related as well as patient-related factors is, therefore, absolutely mandatory. Because of the reduction of therapy-associated adverse events, mainly by better supportive therapies and the introduction of reduced RIC, the indication has been extended within the last years even to patients in their seventh decade of life. In contrast, hypomethylating agents (HMAs) (azacitidine, decitabine) have become available and effective drugs, with azacitidine (AZA) being superior compared with conventional care.
Whether patients with advanced MDS actually benefit from an allogeneic SCT has only been studied in retrospective analyses with varying results ( Table 3 ). Prospective studies are lacking so far, but several studies have been initiated to compare allogeneic SCT and nontransplant among older patients with MDS.
| n | Age (y) | Conditioning | |
|---|---|---|---|
| Yes | |||
| Koreth et al, 2013 | 184 | 60–70 | RIC |
| Alessandrino et al, 2013 | 1109 | 46–63 | RIC/MAC |
| Platzbecker et al, 2012 | 178 | 63–66 | RIC/MAC |
| Cutler et al, 2004 | 674 | 39–50 | MAC |
| No | |||
| Jabbour et al, 2013 | 93 | 51–54 | MAC |
| Brand et al, 2013 | 384 | 55–69 | RIC/MAC |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree