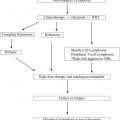
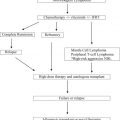
Allogeneic stem cell transplantation (HSCT) offers the only potentially curative approach in chronic lymphocytic leukemia (CLL). However, this applies only to a minority of patients, and is associated with significant treatment-related mortality and morbidity. HSCT must therefore always be considered in view of other, potentially less toxic therapies. Several new agents demonstrate impressive and durable responses in high-risk patients who might be candidates for HSCT. Therefore the choice of HSCT versus a novel agent is one that must be gauged on a patient-by-patient basis; this will change as data mature on the use of these novel agents in CLL.
Key points
- •
Hematopoietic stem cell transplantation (HSCT) offers the only potentially curative approach to the treatment of chronic lymphocytic leukemia (CLL) but is suitable only for a minority of patients and is associated with significant treatment-related mortality and morbidity.
- •
Guidelines suggest that HSCT is indicated in fit CLL patients with a suitable matched donor, del17p-/TP53 mutations, or who have relapsed shortly after chemo-immunotherapy (high-risk patients).
- •
HSCT must always be considered in view of other, potentially less toxic therapies.
- •
Several new agents demonstrate impressive and durable responses in high-risk patients who might be candidates for transplant.
- •
The choice of HSCT versus a novel agent is one that must be gauged on a patient-by-patient basis.
Introduction: how the availability of immunochemotherapy and novel substances are changing Chronic lymphocytic leukemia treatment
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the Western world and is characterized by the progressive accumulation of mature typically CD5-positive B lymphocytes within the blood, bone marrow, and secondary lymphoid organs. Although CLL is mostly an indolent disease, there are subgroups of patients that die within a few years from diagnosis despite intensive therapy. Over the past decade, significant advances in the understanding of the pathogenesis of CLL have led to the development of a range of novel treatment options for patients requiring therapy. In young patients without significant comorbidities, immunochemotherapy with fludarabine, cyclophosphamide, and the anti-CD20 monoclonal antibody (mAb) rituximab (FCR) has been established as the first-line standard-of-care treatment. Although this regimen leads to high overall response rates (ORR) and a long progression-free survival (PFS), it is unsuitable for certain subgroups of patients: these include patients with p53 abnormalities who respond poorly to purine-analogue-based immunochemotherapy and relapse often and early, and elderly patients with comorbidities unable to tolerate FCR-associated toxicities.
In the latter, chlorambucil is a widely accepted therapeutic option, and the combination with rituximab is generally well tolerated and improves PFS. A recently published pivotal phase 3 trial by the German CLL Study Group showed that the type 2 anti-CD20 antibody obinutuzumab was superior to rituximab when each was combined with chlorambucil. Ofatumumab is another fully humanized anti-CD20 mAb that has revealed high efficacy in untreated and relapsed/refractory patients, and even in patients pretreated with rituximab. Several recent clinical studies indicate that novel agents interfering with B-cell receptor (BCR) signaling, such as the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, the PI3kp110δ inhibitor idelalisib, or the BCL2 inhibitor navitoclax, are well tolerated and very active, even for the treatment of relapsed and fludarabine-refractory CLL, and various combinations with immunochemotherapy are currently being tested in registration studies or are under clinical development. Although these early results appear very encouraging, it is yet to be seen how they will translate into long-lasting remissions and disease control. In addition, a recent report indicates that patients can become resistant to ibrutinib therapy because of mutations of drug binding sites within the BCR pathway, and similar resistance mechanisms to other substances are likely.
The only curative treatment option in CLL so far is allogeneic hematopoietic stem cell transplantation (HSCT). HSCT takes advantage of the graft-versus-leukemia (GvL) effect mediated by differentiated transplanted effector cells, which are capable of mounting an antitumor immune response and inducing long-lasting clinical remission. However, HSCT is only suitable for a selected group of patients, and the challenges that HSCT has to face in 2014 are the following:
- •
To identify and predict which patients and specific subgroups of patients benefit most from HSCT, and in which novel substances are unlikely to alter the biological course of their disease
- •
To recognize the appropriate time point when HSCT should be offered
- •
To determine if and how HSCT should be best combined with novel therapeutic options.
This review summarizes the current knowledge on HSCT in CLL and critically discusses its role in the era of novel treatment strategies.
Introduction: how the availability of immunochemotherapy and novel substances are changing Chronic lymphocytic leukemia treatment
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the Western world and is characterized by the progressive accumulation of mature typically CD5-positive B lymphocytes within the blood, bone marrow, and secondary lymphoid organs. Although CLL is mostly an indolent disease, there are subgroups of patients that die within a few years from diagnosis despite intensive therapy. Over the past decade, significant advances in the understanding of the pathogenesis of CLL have led to the development of a range of novel treatment options for patients requiring therapy. In young patients without significant comorbidities, immunochemotherapy with fludarabine, cyclophosphamide, and the anti-CD20 monoclonal antibody (mAb) rituximab (FCR) has been established as the first-line standard-of-care treatment. Although this regimen leads to high overall response rates (ORR) and a long progression-free survival (PFS), it is unsuitable for certain subgroups of patients: these include patients with p53 abnormalities who respond poorly to purine-analogue-based immunochemotherapy and relapse often and early, and elderly patients with comorbidities unable to tolerate FCR-associated toxicities.
In the latter, chlorambucil is a widely accepted therapeutic option, and the combination with rituximab is generally well tolerated and improves PFS. A recently published pivotal phase 3 trial by the German CLL Study Group showed that the type 2 anti-CD20 antibody obinutuzumab was superior to rituximab when each was combined with chlorambucil. Ofatumumab is another fully humanized anti-CD20 mAb that has revealed high efficacy in untreated and relapsed/refractory patients, and even in patients pretreated with rituximab. Several recent clinical studies indicate that novel agents interfering with B-cell receptor (BCR) signaling, such as the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, the PI3kp110δ inhibitor idelalisib, or the BCL2 inhibitor navitoclax, are well tolerated and very active, even for the treatment of relapsed and fludarabine-refractory CLL, and various combinations with immunochemotherapy are currently being tested in registration studies or are under clinical development. Although these early results appear very encouraging, it is yet to be seen how they will translate into long-lasting remissions and disease control. In addition, a recent report indicates that patients can become resistant to ibrutinib therapy because of mutations of drug binding sites within the BCR pathway, and similar resistance mechanisms to other substances are likely.
The only curative treatment option in CLL so far is allogeneic hematopoietic stem cell transplantation (HSCT). HSCT takes advantage of the graft-versus-leukemia (GvL) effect mediated by differentiated transplanted effector cells, which are capable of mounting an antitumor immune response and inducing long-lasting clinical remission. However, HSCT is only suitable for a selected group of patients, and the challenges that HSCT has to face in 2014 are the following:
- •
To identify and predict which patients and specific subgroups of patients benefit most from HSCT, and in which novel substances are unlikely to alter the biological course of their disease
- •
To recognize the appropriate time point when HSCT should be offered
- •
To determine if and how HSCT should be best combined with novel therapeutic options.
This review summarizes the current knowledge on HSCT in CLL and critically discusses its role in the era of novel treatment strategies.
The unmet need of poor-risk chronic lymphocytic leukemia patients before the availability of novel substances
Although immunochemotherapy has significantly improved the outcome for most CLL patients, there are subgroups of patients who have repeatedly been identified as having a poor response to therapy. The pivotal report by Döhner and colleagues predicted that patients with del17p- typically require therapy within 1 year of diagnosis and have a median overall survival (OS) of just 32 months. This lack of chemosensitivity is biologically explained by the malfunction of the tumor suppressor protein p53, whose gene locus is located on the short arm of chromosome 17. In CLL, deletion of 17p- leads to the inactivation of the TP53 gene; this is often accompanied by inactivating mutations of the second locus of TP53, leading to a complete loss of function. Within the pivotal CLL8 trial, which demonstrated the superiority of frontline FCR over fludarabine and cyclophosphamide alone, del17p- was the strongest negative predictive factor for response to therapy and survival, and the clinical responses that were achieved were not durable. Even though there are retrospective data indicating that some patients with del17p- might experience an indolent course despite the mutation, similar unfavorable outcomes have been observed in other prospective trials using combinations of rituximab with bendamustine or fludarabine alone.
Until recently, the only therapy that appeared to be able to overcome the negative impact of p53 abnormalities in the first-line treatment setting, in terms of both its predictive value and its effect on the response to treatment, is the anti-CD52 mAb alemtuzumab and combinations with chlorambucil, high-dose corticosteroids, rituximab, and FCR. These approaches, however, are associated with high hematological and nonhematological toxicities and severe infectious complications and are therefore unsuitable for most elderly CLL patients. In the relapsed setting, the management of patients with TP53 abnormalities is even more challenging. Several clinical studies have demonstrated that FCR and combinations with high-dose corticosteroids, alemtuzumab, or alternative regimens consisting of rituximab, oxaliplatin, cytarabine, and fludarabine (OFAR) have only limited and short-term efficacy and are associated with high toxicity rates. However, depending on doses and application routes, it also seems feasible that alemtuzumab-based regimens can serve as a means to “bridge” the time to HSCT.
Summary
del17p- and/or p53 abnormalities are negative predictive factors for response to therapy and survival. These abnormalities can be partly overcome by treatment with the anti-CD52 antibody alemtuzumab, alone and in combination, but it comes at the cost of high nonhematological and hematological toxicities.
Indication of transplantation: the 2007 European Society of Blood and Marrow Transplantation consensus criteria and beyond
In line with the experiences from these (immuno)chemotherapy-based clinical trials, the European Society of Blood and Marrow Transplantation (EBMT) transplant consensus from 2007 states that HSCT is a reasonable treatment option in relapsed/fludarabine-refractory patients and patients with p53 abnormalities with indication for treatment. This option is also reflected in the 2008 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines, which recommend that patients with resistant disease, a short time to progression, and del(17p) should be offered investigative clinical protocols, including HSCT. In a more recently published Perspective , 3 risk categories were suggested based on the predicted effectiveness of FCR-like treatment, and the “highest-risk” category included patients in which treatment with FCR is unlikely to yield acceptable response or remission rates or prolong survival. Features of “highest-risk” include TP53 loss/mutation, purine analogue-refractoriness, a very short response to prior FCR, and failure to achieve complete response (CR) after FCR; these patients were considered prime candidates for investigational agents in clinical trials and HSCT. These definitions are summarized in Table 1 .
| EBMT criteria Dreger et al, 2007 |
|
| iwCLL criteria Hallek et al, 2008 |
|
| Highest risk in risk category model Zenz et al, 2012 |
|
Summary
Internationally accepted guidelines suggest that HSCT is indicated in patients who are fit enough for this approach, have a suitable matched donor, have 17p deletion or TP53 mutations, or have relapsed relatively quickly after chemo-immunotherapy.
Evidence for the efficacy of hematopoietic stem cell transplantation in chronic lymphocytic leukemia
The first myeloablative treatment-based transplantation strategies were developed more than 20 years ago, but were unsuitable for most patients because of their high morbidity and mortality. After it was recognized that engraftment and GvL activity can be achieved without preceding myeloablative treatment, nonmyeloablative reduced intensity conditioning (RIC) strategies have made HSCT accessible to a larger cohort of CLL patients, including the elderly and those with comorbidities, reflected in the number of RIC-HSCTs in the EBMT registry: according to the 2012 annual activity survey, 3% of all HSCT indications were performed in CLL (n = 475), mostly from unrelated donors, making CLL the most frequent indication for HSCT among lymphomas.
Several large studies have demonstrated that in contrast to other intensive therapies, the relapse incidence after HSCT seems to decrease over time, indicating that RIC HSCT provides long-term disease control in about 40% of patients and also overcomes the negative prognostic effect of p53 abnormalities and fludarabine-refractoriness. The results from the largest reported prospective studies are summarized in Table 2 . The curative potential of HSCT was also confirmed in patients with SF3B1 and NOTCH1 gene mutations, which have been identified as novel recurrent genetic mutations in CLL and are mostly associated with resistance or poor response to conventional treatment. A smaller, recently published prospective trial of 40 patients using RIC with fludarabine, total body irradiation, and rituximab demonstrated a positive effect of rituximab on OS and EFS in multivariate analyses.
| Fred Hutchinson Cancer Center | German CLL Study Group | MD Anderson Cancer Center | Dana Farber Cancer Institute | |
|---|---|---|---|---|
| Sorror et al, 2008 | Dreger et al, 2010 & 2013 | Khouri et al, 2011 | Brown et al, 2013 | |
| Number of patients | 82 | 90 | 86 | 76 |
| Conditioning regimen | Flu/low-dose TBI | Flu/Cy ± ATG | Flu/Cy ± R | Flu/Bu |
| Donors, % (sibling/MUR) | 63/37 | 41/59 | 50/50 | 37/63 |
| Median follow-up, mo | 60 | 72 | 37 | 61 |
| Early mortality, % (<100 d) | <10 | <3 | <3 | <3 |
| NRM, % | 23 | 23 | 17 | 16 |
| Acute grade 3-4 GvHD, % | 20 | 14 | 7 | 17 |
| Severe chronic GvHD, % | 53 | 55 | 56 | 48 |
| Median PFS, % | 39 (5 y) | 38 (6 y) | 36 (6 y) | 43 (6 y) |
| Median OS, % | 50 (5 y) | 58 (6 y) | 51 (6 y) | 63 (6 y) |
HSCT seems particularly active in patients with complete or partial disease remission at the time of transplantation: in patients with chemosensitive disease, the 5-year OS could be increased to up to 80%. To achieve a good remission state is challenging however, and as discussed before, some regimens that seem suitable happen at the cost of high toxicities. Recent studies indicate that modified OFAR and alemtuzumab-based regimens are the most favorable strategies to help prepare patients for successful HSCT by achieving good remissions. In general, pretransplant characteristics as assessed by the EBMT risk score seem to be of predictive value for OS: this score uses 5 patient-specific pretransplant variables (age, disease status, time from diagnosis to transplant, donor type, and donor–recipient sex combination). A retrospective EBMT analysis demonstrated that there was a significant difference in OS at 5 years between patients with score 1 to 3 and patients having a higher score and also supported the use of matched unrelated donors as equivalent alternative to HLA-matched sibling donors in CLL.
Summary
RIC HSCT provides long-term disease control in about 40% of patients, including patients with adverse prognostic markers, but remission status at the time of transplantation and pretransplant characteristics are predictive of HSCT outcome.
Posttransplantation monitoring by minimal residual disease kinetics and graft-versus-leukemia activity
The curative effect of HSCT in some patients supports our current understanding of the importance of minimal residual disease (MRD) as a quantification of treatment response. MRD denotes a subclinical disease burden that remains after specific therapy. For CLL, this is defined as a contamination of 5 CLL cells or less per nanoliter of peripheral blood in the absence of clinical signs or symptoms of the disease. Patients showing less than one CLL cell in 10,000 benign leukocytes in peripheral blood or bone marrow are considered MRD-negative. MRD levels have been demonstrated to be an independent predictor of PFS and OS after immunochemotherapy and add significantly to the prognostic power of known pretreatment parameters. Recent data indicate that they can potentially also be used to de-escalate treatment based on the MRD depth of response. After HSCT, MRD kinetics rather than levels seem to identify patients that are at risk of clinical relapse, long before clinical signs become apparent. This is most likely mediated by ongoing GvL activity of donor T lymphocytes and their continuous immunotherapeutic activity, which is highly sensitive to immunomodulation by immune suppression or donor lymphocyte infusions.
Summary
After HSCT, MRD kinetics aid in the assessment of response and indicate the level of ongoing GvL-mediated immunotherapeutic activity.
Adverse events and risk of graft-versus-host disease in chronic lymphocytic leukemia
GvL activity in CLL seems to be closely correlated to graft-versus-host disease (GvHD), as patients with chronic GvHD (cGvHD) have a reduced risk of relapse. Accordingly, an increased relapse rate was observed when donor T cells were depleted. However, cGvHD remains a significant problem and is largely responsible for nonrelapse mortality (NRM) rates of up to 23% and affects up to 60% of patients in the large clinical trials summarized in Table 2 . Apart from its impact on NRM, cGVHD is the major determinant of quality of life after HSCT. The clinical symptoms of cGVHD, however, decrease over time in many affected patients, and therapeutic immunosuppression could be terminated after 1 to 2 years in many patients in the trials summarized in Table 2 .
Other acute side effects of RIC HSCT in the early transplant phase include nausea, mucositis, and infections. Because of substantial improvements of supportive and anti-infective treatments and the availability of dedicated transplant units, these are considerably easier to manage than in the era of myeloablative HSCT, which is reflected in very low early mortality rates of less than 10% in the first 100 days after HSCT (see Table 2 ).
Summary
HSCT is associated with significant treatment-related mortality and morbidity, largely due to chronic GvHD.
Management of relapse after hematopoietic stem cell transplantation
Even though HSCT can be curative in up to 40% of patients, a significant proportion still relapses after HSCT. To date, there is no standard treatment or guidelines available for patients who failed HSCT and are unresponsive to post-HSCT immunomodulation-based interventions. In a retrospective analysis of 40 patients from the MD Anderson Cancer Center (MDACC), median time to HSCT failure was 7 months, and the most common salvage treatment regimens were re-treatment with rituximab-based and alemtuzumab-based immunochemotherapy and treatment with thalidomide or lenalidomide and ibrutinib. This treatment led to a median OS from time of progression of 53 months, indicating that post-HSCT relapses are sensitive to salvage therapy. Interestingly, there were no differences between FCR, alemtuzumab, or combination chemotherapy in OS, whereas most patients that received ibrutinib were still alive at the time of last follow-up.
Summary
The clinical management of relapse after HSCT is challenging but seems to be sensitive to immunochemotherapy treatment.
Autologous hematopoietic stem cell transplantation
Long before the advent of fludarabine or antibody-based strategies, there was realistic hope that myeloablative therapy followed by autologous stem cell transplantation (autoSCT) might be an effective and potentially curative front-line treatment option for suitable patients with CLL. Since then, several prospective trials have demonstrated that autoSCT can prolong EFS and PFS if used as part of early front-line treatment, but fails to improve OS and lacks the potential to overcome the negative impact of biomarkers that confer resistance to chemotherapy or early relapse. In addition, it is associated with increased risk of late adverse events such as secondary malignancies. Therefore, autoSCT currently does not play a role in the treatment of CLL, and patients that have benefited from this approach in the past are also most likely to respond to conventional immunochemotherapy.
Summary
Autologous SCT no longer plays a role in the treatment of CLL.
Outcome of poor risk chronic lymphocytic leukemia patients in the era of novel substances and treatments
Because of the availability of novel substances and treatment strategies, the standard of care in CLL is changed dramatically. These new approaches include new mAbs, immune modulatory agents, substances interfering with the BCR signaling pathway, and novel cellular therapies. As extensive reviews have been published elsewhere, the impact on patients with high-risk CLL is the focus of this section.
Although representing a “passive” immunotherapy, mAbs display enhanced antitumoral activity by engaging the immune system through increased complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity. Anti-CD20 mAbs are now integral components of CLL therapy, but the new generation antibody ofatumumab seems to be more effective in refractory patients and in patients pretreated with rituximab, yielding a response rate of almost 60%. Lenalidomide has been demonstrated to have pleiotropic effects on immune cells in both preclinical and clinical studies, primarily by enhancing antitumoral immunity in effector cells. Several clinical trials have demonstrated that lenalidomide as a single agent and in combination with rituximab has activity both in untreated and relapsed/refractory CLL and in patients with del17p-, but is associated with nonnegligible toxicities such as tumor flare reaction and increased risk of opportunistic infections. The combination of lenalidomide with ofatumumab is currently being investigated in relapsed/refractory CLL.
The most striking results have been observed in recently published prospective trials using the BCR signaling inhibitors ibrutinib and idelalisib: ibrutinib is a BTK inhibitor that primarily blocks BCR associated anti-apoptosis pathways. In addition, it affects BCR-controlled and chemokine-controlled retention and homing of CLL cells in their growth-supporting and survival-supporting lymph node and bone marrow microenvironment. In a phase I/II study in 85 heavily pretreated patients with relapsed or refractory CLL, the ORR was 71%, with a PFS of 75% and an OS of 83% at 26 months, and response was independent of del17p-. Toxicities were mild and only a few serious adverse events were observed. Idelalisib is an inhibitor of PI3K110δ, which is also a component of CLL signaling pathways involved in cell survival, clonal expansion, and malignant cells retention in lymphoid tissues. In a phase I study conducted on 54 heavily pretreated CLL patients with relapsed/refractory disease, including patients with del17p- (24%), 81% of patients achieved nodal responses with an overall response rate of 72% and a very acceptable safety profile. A phase III trial was then initiated on 220 patients with relapsed CLL receiving idelalisib in combination with rituximab versus rituximab plus placebo. Because of overwhelming efficacy, the study was interrupted after the first interim analysis: the ORR was 81% for the combination therapy versus 13% for rituximab monotherapy, whereas OS at 12 months was 92% versus 80%, and PFS was 93% versus 46%. Multiple ongoing randomized and nonrandomized trials are now investigating combinations of these substances with ofatumumab, rituximab, bendamustine, and other novel agents.
BCL-2 antagonists such as navitoclax and ABT-199 mainly work by triggering apoptosis via targeting the BCL2 family. In a phase I study with 29 patients with relapsed/refractory CLL, lymphocytosis was reduced by more than 50% in 19 of 21 patients with baseline lymphocytosis, and partial response (PR) or stabilization of disease was achieved in almost half of the patients, again including patients with del17p- CLL. Another promising group of agents that have shown efficacy in high-risk CLL patients are cyclin-dependent kinase inhibitors such as flavopiridol. In a recently published phase I study, the combination with cyclophosphamide and rituximab was tolerable and active in high-risk CLL patients without being associated with tumor lysis syndrome, confirming previous findings that flavopiridol can overcome the negative impact of del17p-.
However, a recent report indicates that patients can become resistant to ibrutinib therapy because of mutations of drug-binding sites within the BCR pathway, and similar resistance mechanisms to other substances are likely are currently under investigation.
A very exciting new active immunotherapy strategy is chimeric antigen receptor (CAR) T-cell therapy. CAR technology has recently emerged as a novel and promising perspective to specifically target malignant cells with precisely engineered T cells. It uses the single-chain variable fragment from an antibody molecule fused with an internal T-cell signaling domain to form a CAR, which is then transduced into T cells. A major advantage of this approach is that it eliminates MHC restriction, enabling the same CAR to be used for several different patients. In a pivotal report, a heavily pretreated high-risk patient with refractory CLL received autologous T cells that had been modified with CARs directed at CD19, a B-cell surface antigen, resulting in remission induction and lasting tumor control. Since then, several clinical trials have reported impressive results with anti-CD19 CARs, in both CLL and acute lymphoblastic leukemia. However, it has also become clear that the success of CAR therapy depends on the inclusion of lympho-reducing conditioning chemotherapy and the choice of CAR design. In addition, CAR T-cell therapy can be associated with severe complications such as cytokine release syndrome, a potentially lethal complication, and lasting normal B-cell depletion.
Summary
There are several new agents and immunotherapy approaches that are in clinical trials or recently approved in CLL that demonstrate impressive responses and durable durations of response in high-risk patients who might be candidates for transplant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree