Acute myeloid leukemia (AML) is associated with poor outcome mainly because of relapse. The best antileukemic treatment is allogeneic stem cell transplantation. However, the associated significant nonrelapse mortality limits both the application and outcome of the procedure. Recent advances in understanding the genetic landscape of the disease enable educated selection of patients. Improved treatment protocols, supportive therapy, patient selection, and posttransplant manipulations all contribute to a better outcome.
Key points
- •
Allogeneic stem cell transplantation (allo-SCT) is recommended for patients with intermediate and unfavorable genetic risk.
- •
Allo-SCT is best performed in CR1. The chance of achieving CR2 is only 50%.
- •
Matched unrelated donor is increasingly used with no difference in the outcome compared with matched related donor (MRD).
- •
Alternative donors such as cord blood (CB) and haploidentical are being used, with encouraging data. There is no recommendation regarding the preferable stem cell source.
- •
Autologous SCT has a potent antileukemic effect mainly in favorable and intermediate-risk cytogenetic groups with reduced relapse and better leukemia-free survival compared with chemotherapy.
- •
Allo-SCT can safely be administered to fit older adults (aged 60–75 years), with results similar to those of younger adults.
- •
Further improvement in allo-SCT outcome includes assessment of minimal residual disease, graft engineering and incorporation of novel approaches (vaccines, adoptive T-cell transfer, and targeted therapy).
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by somatic acquisition of genetic and epigenetic alterations in hematopoietic myeloid progenitors that perturb normal mechanisms of self-renewal, proliferation, and differentiation through accumulation of multistep cooperating mutations. Advance in molecular methods showed 23 commonly mutated genes and an average of 13 mutations per patient. The heterogeneity is not only interpatient but also intrapatient, with concomitant presence of genetically different leukemic subclones. This intraclonal genetic diversity reflects natural selection, leading to clonal evolution, disease progression, and relapse. In addition, it was recently shown that the disease may originate from a preleukemic hematopoietic progenitor harboring the DNMT3 or IDH2 mutations, which confers clonal expansion and with additional mutations can transform into AML. The importance of these findings is that to cure leukemia, we need to address not only the dominant clone at disease onset but also minor subclones that can lead to relapse. Until we better define the disease at the genetic level and its origin, AML treatment is intended to achieve complete remission (CR) with induction followed by postremission consolidation with either intensive chemotherapy, autologous stem cell transplantation (ASCT) or allogeneic stem cell transplantation (allo-SCT). This review focuses on the current role of transplantation in AML.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by somatic acquisition of genetic and epigenetic alterations in hematopoietic myeloid progenitors that perturb normal mechanisms of self-renewal, proliferation, and differentiation through accumulation of multistep cooperating mutations. Advance in molecular methods showed 23 commonly mutated genes and an average of 13 mutations per patient. The heterogeneity is not only interpatient but also intrapatient, with concomitant presence of genetically different leukemic subclones. This intraclonal genetic diversity reflects natural selection, leading to clonal evolution, disease progression, and relapse. In addition, it was recently shown that the disease may originate from a preleukemic hematopoietic progenitor harboring the DNMT3 or IDH2 mutations, which confers clonal expansion and with additional mutations can transform into AML. The importance of these findings is that to cure leukemia, we need to address not only the dominant clone at disease onset but also minor subclones that can lead to relapse. Until we better define the disease at the genetic level and its origin, AML treatment is intended to achieve complete remission (CR) with induction followed by postremission consolidation with either intensive chemotherapy, autologous stem cell transplantation (ASCT) or allogeneic stem cell transplantation (allo-SCT). This review focuses on the current role of transplantation in AML.
Indications for stem cell transplantation in the genomic era
Evaluation of AML prognosis shifted over the last 2 decades from clinical (or patient related) to a more powerful biological one, based on cytogenetic and molecular alterations present in DNA blasts at diagnosis (disease related). Although under constant revision, biological prognosis is subdivided into 3 main categories of favorable, intermediate, and poor risk. Integrated AML-related prognostic parameters are presented in Table 1 .
| Cytogenetic Markers | Molecular Markers | Clinical Factors |
|---|---|---|
| t (8;21) inv(16)/t (16;16) t (15;17) | Mutated CEBPA (double) Mutated NPM1 (without FLT3 –ITD mutation) | Minimal residual disease negative |
| Adverse prognostic factors | ||
| inv(3)/t (3;3) t (9;22) t (9;11) t (6;9) −5 or del (5q) −7 abn (17p) Complex karyotype Monosomal karyotype | Enhanced Evi-1 expression MLL rearrangements FLT3 –ITD mutation DNMT3A mutation BAALC expression ERG expression MN1 expression WT1 polymorphism BCR–ABL -positive | Increased age Increased WBC count Extramedullary disease No early CR Persistent minimal residual disease CD34 + blasts Treatment-related AML |
The recommended LeukemiaNet reporting on AML prognosis was developed based on large retrospective studies, such as the Medical Research Council (MRC). However, the postremission treatment was not stratified. The prognostic significance of these genetic alterations was recently evaluated in a large cohort of non–transplant treated patients. The classification clearly separates different genetic groups by outcome and hence can be used for stratification into different treatment groups in clinical trials.
Integrated genetic profiling is a step forward in AML risk assessment. Using mutational analysis of 18 genes known to be involved in AML, Patel and colleagues managed to further refine the large intermediate-risk cytogenetic group into 3 significantly different prognostic subgroups based on molecular alterations, thus emphasizing the significance of coexistence and cooperation of different mutations in AML ( Figs. 1–3 ).
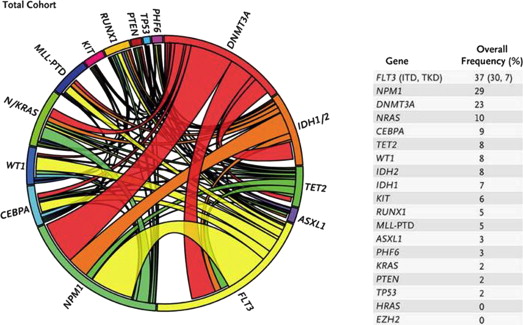
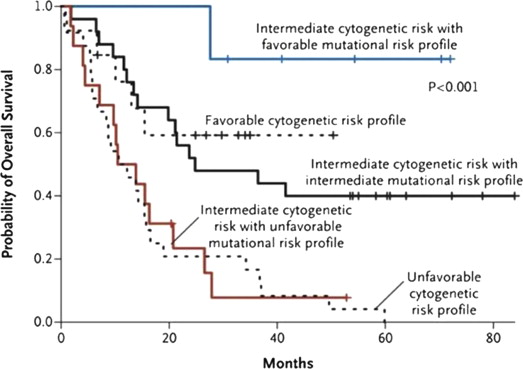
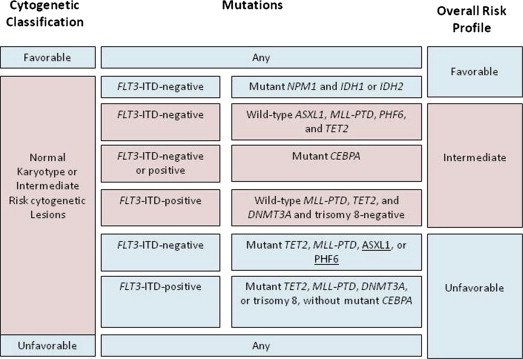
Without diminishing the importance of prognostic scores, the predictive value of postremission therapy should be cautiously interpreted, because it has not been prospectively tested in relation to postinduction treatment assignment. In addition, rapidly evolving knowledge and complexity of cooperating genetic alterations dissect patient populations into small cohorts, making analysis more complicated.
Yet, available data, mainly from retrospective subanalyses of patients in large study groups that were genetically randomized to donor versus no donor, can aid in decision making. Patients with the FLT3-ITD mutation have a CR rate similar to that observed in patients with the wild-type after induction therapy; however, they have a higher relapse rate (RR). Most studies, although retrospective, reported that patients harboring the mutation had a better disease-free survival (DFS) and overall survival (OS) after allo-SCT compared with chemotherapy only. The beneficial effect of allo-SCT was restricted in some of the studies to patients having a low allelic ratio of mutated to wild-type FLT3 less than 0.8 or less than 0.5. In contrast, other studies failed to show an improved outcome after allo-SCT, whereas a large registry European Group For Blood and Marrow Transplantation (EBMT) study reported inferior outcome after allo-SCT in FLT3-positive versus FLT3-negative patients.
Monosomal karyotype is known to be associated with a poor outcome in AML. Whether it retains its prognostic effect after allo-SCT is not clear. Although some studies reported an improved outcome, especially when the disease was not associated with complex karyotype, others reported no or limited beneficial effect from a transplant.
In the absence of prospective studies, a matched-pair analysis of prospectively treated patients can better clarify the role of allo-SCT in different cytogenetic groups. In a study by the German AML Cooperative Group (AMLCG99), allo-SCT (both from related and unrelated donors) had a significantly superior OS and decrease in RR compared with chemotherapy in both intermediate and unfavorable groups.
Meta-analysis of the role of allo-SCT in AML can aid in decision making, because of the attainment of significant statistical power when data on many patients are combined. Studies by Koreth and colleagues including 6007 patients with AML in CR1 and by Cornelissen and colleagues with 1033 patients from 4 large intergroup trials showed improved leukemia-free survival (LFS) and OS in both poor-risk and intermediate-risk groups.
When considering allo-SCT in AML, it is imperative to include covariates of the transplant itself, such as patient age, comorbidities, availability of a matched related or unrelated donor, and transplant regimen. These data accumulated in the LeukemiaNet recommendations on allo-SCT in AML patients in CR1 are presented in Table 2 .
| AML Risk Group b | AML Risk Assessment c | Risk of Relapse After Consolidation Approach | Prognostic Scores for Nonrelapse Mortality that Would Indicate Allogeneic HSCT as Preferred Consolidation | |||
|---|---|---|---|---|---|---|
| Chemotherapy or AHSCT (%) | Allo-HSCT (%) | EBMT Score | HCT–CI Score | Nonrelapse Mortality Risk (%) | ||
| Good | t (8;21) with WBC ≤20 Inv(16)/t (16;16) Mutated CEBPA (double allelic) Mutated NPM1 (No FLT3– ITD mutation) Early first CR and no minimal residual disease | 35–40 | 15–20 | NA (≤1) | NA (<1) | 10–15 |
| Intermediate | T (8;21) with WBC >20 Cytogenetically normal (or with loss of X and Y chromosomes), WBC count ≤100 and early first CR (after first cycle of chemotherapy) | 50–55 | 20–25 | ≤2 | ≤2 | <20–25 |
| Poor | Otherwise good or intermediate, but no CR after first cycle of chemotherapy Cytogenetically normal and WBC >100 Cytogenetically abnormal | 70–80 | 30–40 | ≤3–4 | ≤3–4 | <30 |
| Very poor | Monosomal karyotype Abn3q26 Enhanced Evi-1 expression | >90 | 40–50 | ≤5 | ≤5 | <40 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree