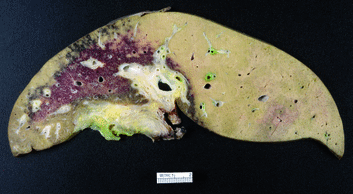
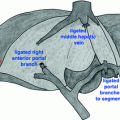
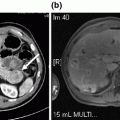
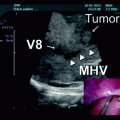
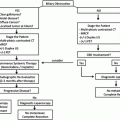
Fig. 20.1
Mayo Clinic Protocol. Neoadjuvant therapy is targeted to the primary tumor and regional lymph nodes. EBR, external beam radiation. Intraluminal brachytherapy with iridium is administered through an endoscopically placed biliary tube. LDLT, living donor liver transplant. DDLT, deceased donor liver transplant [8, 9]
Table 20.1
Criteria for neoadjuvant therapy and liver transplantation
Criteria for neoadjuvant therapy and liver transplantation | |
|---|---|
1. Diagnosis of cholangiocarcinoma • Transcatheter biopsy or brush cytology • CA 19-9 > 100 U/mL or mass on cross-sectional imaging with a malignant-appearing stricture on cholangiography • Biliary ploidy by FISH with a malignant-appearing stricture on cholangiography 2. Unresectable tumor above the cystic duct • Pancreaticoduodenectomy for microscopic involvement of the CBD • Resectable CCA arising in PSC 3. Radial tumor diameter ≤ 3 cm 4. Absence of intra- and extrahepatic metastases 5. Candidate for liver transplantation |
CASE 1
A 61-year-old male with no significant medical history other than hyperlipidemia presented with jaundice and a cholestatic liver profile (total bilirubin 3.3 mg/dL, alkaline phosphatase 350 U/L). Initial workup included a normal ultrasound that did not show any ductal dilatation or intrahepatic masses. His symptoms were attributed to drug-induced (atorvastatin) cholestatic hepatitis. Despite discontinuation of the medication, the patient had progression of his cholestatic profile, a 20-pound weight loss, and developed mild epigastric and back pain over the next 2 months. He also developed pruritus, acholic stools, and dark urine.
A malignant-appearing hilar bile duct stricture was finally detected by endoscopic retrograde cholangiography (ERC), and magnetic resonance imaging (MRI) subsequently demonstrated a type IIIB de novo hilar cholangiocarcinoma involving the midportion of the common hepatic duct with extension into the left duct. In addition to mild atrophy of the left liver with compensatory hypertrophy of the right liver, there was concern for encasement of the right hepatic artery. Given these findings, it was felt that resection was not an option, and the patient was referred for evaluation for neoadjuvant therapy and liver transplantation. He was found to be a candidate (see Table 20.1) and completed neoadjuvant radiotherapy with chemosensitization (see Fig. 20.1).
The patient did not have a suitable living donor and was placed on the deceased donor liver waiting list. As time neared for deceased donor organ availability, he underwent hand-assisted laparoscopic staging. The staging operation was negative, and the patient underwent liver transplantation with a liver from a 70-year-old deceased donor. Use of the extended criteria donor enabled him to undergo transplantation several months sooner than would have been possible with a standard criteria donor. Liver transplantation was performed with a standard caval-sparing hepatectomy and end-to-side caval implantation. The hepatic artery was reconstructed with an aortic jump graft using a younger donor iliac artery (Fig. 20.2). Biliary reconstruction was performed with Roux-en-Y choledochojejunostomy (Fig. 20.3).
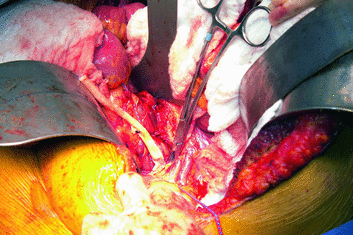
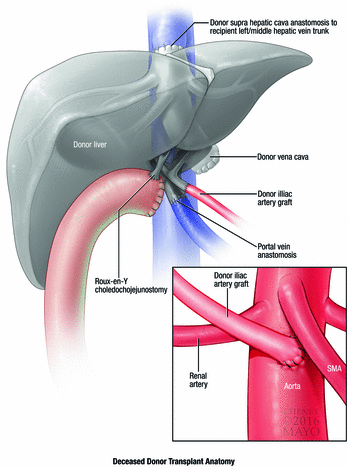

Fig. 20.2
Aortic jump graft. For deceased donors, an infrarenal aortic jump graft is created using donor iliac artery

Fig. 20.3
Deceased donor liver transplant for cholangiocarcinoma. A Roux-en-Y choledochojejunostomy is created for biliary reconstruction, and an infrarenal aortic graft is created for hepatic artery inflow. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved
Intraoperative Pearls
- 1.
Avoidance of dissection in the liver hilus
- 2.
Increased risk of early and late hepatic artery thrombosis
- 3.
Biliary Roux limb reconstruction
Choledochojejunostomy in a deceased donor allograft
Hepaticojejunostomy in a living donor allograft
- 4.
Increased risk of late portal vein stenosis
- 5.
Pancreaticoduodenectomy for distal common bile duct involvement
En bloc pancreaticoduodenectomy without division of the common bile duct in patients with known common bile duct involvement detected by preoperative cytology or biopsy
Reconstruction with a double jejunal limb in living donors separating the biliary and pancreatic anastomoses
Non-pylorus-preserving distal gastrectomy secondary to radiation exposure.
Discussion
The risk of cholangiocarcinoma in PSC patients ranges from 7 to 13%. Due to this risk, the majority of PSC patients undergo routine surveillance cholangiography [13]. In contrast, patients with cholangiocarcinoma arising de novo are frequently diagnosed when they develop jaundice later after the onset of nonspecific symptoms. De novo cholangiocarcinoma is often diagnosed at a more advanced stage of disease than cholangiocarcinoma arising in PSC. Indeed, in the Mayo Clinic experience, de novo patients are more likely to have a mass visible on cross-sectional imaging. Thus, it is not surprising that the results with neoadjuvant therapy and liver transplantation are better for patients with cholangiocarcinoma arising in PSC than those with de novo disease. Current 2-year-survival rates at Mayo Clinic are 60% for PSC patients and 30% for de novo patients after the start of neoadjuvant therapy, and 77 and 56% after liver transplantation. The differences in survival are likely due to earlier detection in PSC patients, since there were no significant differences in survival for de novo cholangiocarcinoma patients following adjustment for patient age, lymph node metastases, and tumor size [14]. Due to limited donor resources and equivocal results compared to resection, neoadjuvant therapy and liver transplantation is not the preferred treatment for de novo patients with resectable disease (Table 20.2) [15]. In contrast, patients with underlying PSC have a predisposition to multifocal cancer; they often have underlying parenchymal disease and/or cholangiopathy such that they are best treated by neoadjuvant therapy and liver transplantation regardless of tumor resectability.
Table 20.2
Criteria for unresectability of non-metastatic hilar cholangiocarcinoma
Criteria for unresectability of non-metastatic hilar cholangiocarcinoma | |
|---|---|
1. Bilateral segmental duct extension 2. Unilateral atrophy with either contralateral segmental ductal or vascular inflow involvement 3. Unilateral segmental ductal extension with contralateral vascular inflow involvement |
In preparation for liver transplantation, all patients should undergo operative staging following completion of neoadjuvant therapy. Operative staging is best performed the day prior to living donor liver transplantation, such that it has minimal impact on technical difficulty and patient recovery. Patients awaiting deceased donor liver transplantation should undergo operative staging as the time nears for donor organ availability. Timing of the operation depends on ABO-specific MELD scores for patients undergoing deceased donor transplantation and will vary between transplant center willingness to accept extended criteria donors, donor service areas, and UNOS regions in the United States. MELD exception scores are granted by UNOS regional review boards for patients with cholangiocarcinoma which fulfill UNOS diagnosis guidelines and are enrolled in a neoadjuvant therapy protocol. MELD scores for cholangiocarcinoma begin at 22 and increase to a score representing a 10% increase in risk every 3 months, a score increase identical to that for hepatocellular carcinoma (22, 25, 27, 29, 31, 33, etc.).
Operative staging was done in an open fashion at our institution between 1993 and 2005. Since 2005, we have been able to accomplish most staging operations using hand-assisted laparoscopy. The hand port is placed through a right subcostal incision, which is later incorporated in the bilateral subcostal incision required for liver transplantation. Operative staging involves thorough intraperitoneal examination with biopsy of any suspicious peritoneal nodules or intrahepatic nodules, excision of the hepatic artery lymph node overlying the takeoff of the gastroduodenal artery, excision of a pericholedochal lymph node usually found posterior to the bile duct, and excision of any suspicious lymph nodes within the hepatoduodenal pedicle. If patients were to have had a cholecystectomy shortly before diagnosis of cholangiocarcinoma (which is not infrequent), the cystic duct stump should be excised for pathological examination. Clinical factors associated with a high likelihood of positive staging and/or patient dropout prior to transplantation include a CA 19-9 ≥ 500 U/L, mass ≥3 cm in radial diameter, and a biologic MELD ≥20 [16].
Although initially controversial, the use of deceased donors for treatment of cholangiocarcinoma has gained acceptance and is necessary for patients who do not have a suitable living donor. Despite being granted MELD exception points, cholangiocarcinoma patients remain disadvantaged and at risk for dropout while awaiting a deceased donor organ. As a result, marginal, high risk, and other extended criteria donor livers are often accepted for these patients to enable transplantation sooner than would otherwise be possible.
In the past, we used donor livers procured after circulatory arrest (donation after cardiac death, DCD). DCD livers have a high risk for cholangiopathy, which can lead to recurrent and severe cholangitis and require retransplantation in up to 30% of patients [17, 18]. We have been able to achieve a 5.9% risk of cholangiopathy by using experienced staff surgeons for DCD procurement and minimizing preservation time, but only for patients amenable to duct-to-duct or choledochoduodenostomy biliary reconstruction. Despite our efforts, we still have a 30% incidence of cholangiopathy with cholangiocarcinoma patients that require Roux-en-Y choledochojejunostomy (due to resection of the recipient duct and irradiation of the duodenum). We now avoid use of DCD donor livers for cholangiocarcinoma patients and reserve their use for patients much less likely to develop cholangiopathy. Older donor livers, however, are excellent grafts for cholangiocarcinoma patients. These donors often have significant iliac artery arteriosclerosis, so a graft from a younger donor should be available for use if necessary.
Technical modifications necessary during liver transplantation for cholangiocarcinoma are outlined below. In order to perform the best possible cancer operation, dissection in the liver hilus is avoided to prevent tumor seeding. Similarly, the hepatoduodenal ligament is dissected close to the duodenum and pancreas with proximal division of the proper hepatic artery and portal vein. Intraoperatively, it can be difficult to differentiate tumor from radiation-induced fibrosis and a generalized desmoplastic response. It can also be very difficult to separate the common bile duct from the portal vein. The portal vein wall can be very friable. Examination of explanted livers has shown that, in most instances, these findings are the result of radiation injury and not tumor infiltration of the soft tissues surrounding the duct (Fig. 20.4).