Chapter 21 Transplantation and Rejection
• Transplantation is the only form of treatment for most end-stage organ failure.
• The barrier to transplantation is the genetic disparity between donor and recipient.
• The immune response in transplantation depends on a variety of factors. Host versus graft responses cause transplant rejection. Histocompatibility antigens are the targets for rejection. Minor antigens can be targets of rejection even when donor and recipient MHC are identical. Graft versus host reactions result when donor lymphocytes attack the graft recipient.
• Rejection results from a variety of different immune effector mechanisms. Hyperacute rejection is immediate and caused by antibody. Acute rejection occurs days to weeks after transplantation. Chronic rejection is seen months or years after transplantation.
• HLA matching is one of two major methods for preventing rejection of allografts. The better the HLA matching of donor and recipient, the less the strength of rejection.
• Successful organ transplantation depends on the use of immunosuppressive drugs. 6-MP, azathioprine, and MPA are antiproliferative drugs. Ciclosporin, tacrolimus, and sirolimus are inhibitors of T cell activation. Corticosteroids are anti-inflammatory drugs used for transplant immunosuppression. Antibodies to the IL2 receptor, or to leukocytes, are widely used.
• The ultimate goal in transplantation is to induce donor-specific tolerance. There is evidence for the induction of tolerance in humans and novel methods for inducing tolerance are being developed. Alloreactive cells can be made anergic. Immune privilege can be a property of the tissue or site of transplant.
• Shortage of donor organs and chronic rejection limit the success of transplantation. Living donation is one way to overcome the shortage of donor organs. Alternative approaches are being investigated. The favored animal for xenotransplantation is the pig.
• the first is that transplantation is an important clinical procedure;
• the second is that transplantation has proved an important tool for understanding immunological mechanisms – for example, the major histocompatibility complex (MHC; see Chapter 5) was first described in the context of transplantation, and transplantation models continue to be widely used as tools in basic as well as applied immunology.
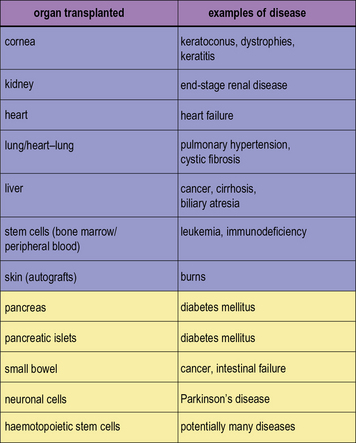
In modern practice many transplants are performed routinely (Fig. 21.1). In addition to routine transplantation of the cornea, kidney, heart, lungs, and liver there is increasing interest in transplanting other organs, such as whole pancreas or islet cells for diabetes mellitus and also small bowel.
Genetic barriers to transplantation
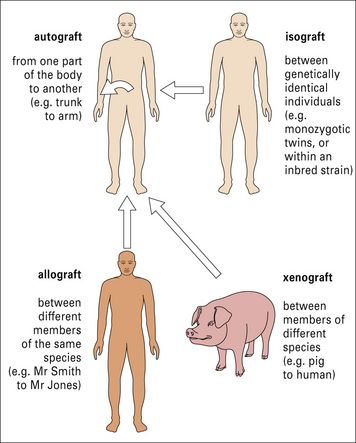
Transplantation is normally performed between individuals of the same species who are not genetically identical, and the antigenic differences are known as allogeneic differences, and result in an allospecific immune response (Fig. 21.2).
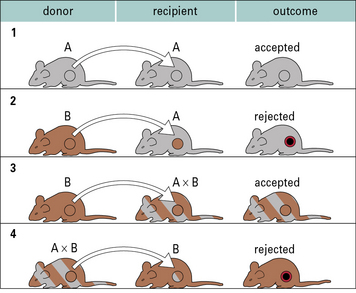
In the case of autografts and isografts there should be no antigenic differences between donor and recipient, and so no immune response. This can be readily illustrated using transplantation of skin or organs between inbred strains of animals (Fig. 21.3).
Graft rejection
Host versus graft responses cause transplant rejection
Q. What are the main genetic differences that are recognized by the host, and why should this be so?
A. Differences in MHC molecule allotypes are most important. The reason is that all nucleated cells express MHC molecules, and the T cell receptor on host T cells has a basic structure that interacts with and recognizes MHC molecules. Also the MHC class I and class II molecules have an extremely high level of genetic variability (see Chapter 5).
The nature of the host versus graft response is discussed in more detail below.
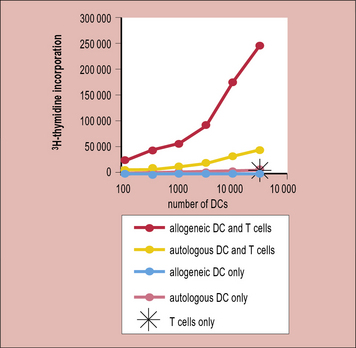
There is a high frequency of T cells recognizing the graft
Thus, in a naive or unimmunized individual fewer than 1/100 000 T cells respond upon exposure to a virus or a protein immunization; however, 1/100–1/1000 T cells respond to allogeneic antigen-presenting cells (APCs). This is reflected in the strong T cell response (proliferation) seen when naive T cells are stimulated with allogeneic dendritic cells (Fig. 21.4)
Histocompatibility antigens are the targets for rejection
Early experiments showed that the bulk of the allospecific response is against molecules of the MHC. We now know that these molecules are the MHC class I or class II molecules (see Chapter 5), which are responsible for presenting antigen (in the form of peptides) to either:
As discussed in Chapter 8, MHC molecules are highly polymorphic, and it is these polymorphic differences that are seen by alloreactive T cells.![]()
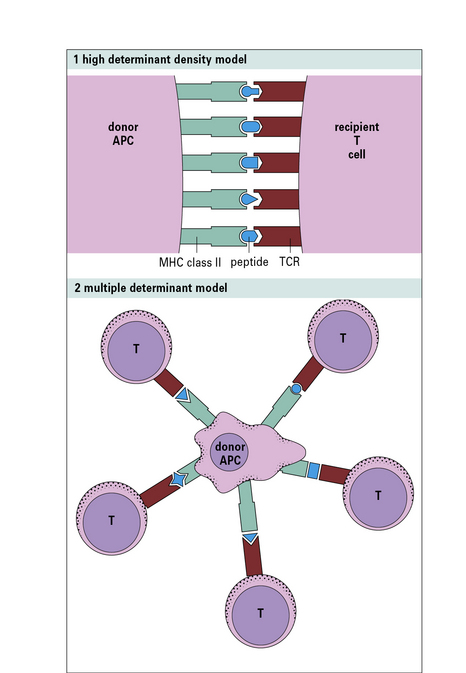
Why is there such a high frequency of allospecific T cells?
The first model (high determinant density model) (Fig. 21.w1) suggests that allospecific T cells recognize the foreign MHC molecules directly, with a low affinity, in a peptide-independent manner. The affinity of the interaction would normally be too low to activate the T cells; however, because the T cells see the MHC molecules directly, and are not recognizing the peptide, this low affinity is compensated for by the high concentration of MHC molecules on allogeneic cells.
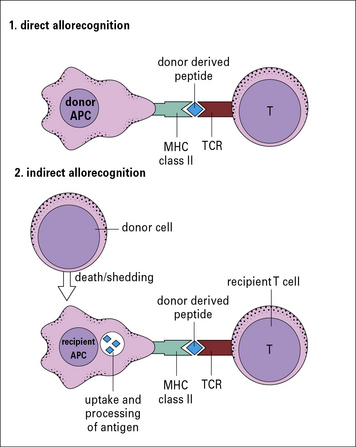
Indirect recognition is important in chronic rejection
In a primary alloresponse (see Fig. 21.4), most of the alloreactive CD4 or CD8 T cells directly recognize the donor MHC molecules.
However, there are other forms of alloresponse, including:
• those against minor MHC antigens (see below); or
• the indirect response in which the recipient CD4 T cells recognize donor MHC molecules that have been processed by recipient APCs and are presented as peptides in the context of recipient MHC class II molecules (Fig. 21.5).
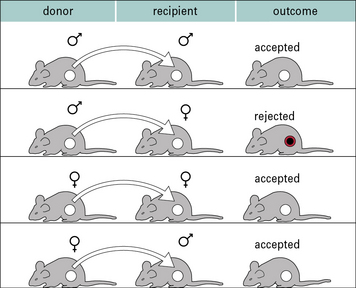
Minor antigens can be targets of rejection even when donor and recipient MHC are identical
Perhaps the best studied minor histocompatibility antigen system is the H-Y system. These are antigens encoded by the Y chromosome, and so are expressed only on male cells. Thus, following immunization, it is possible to demonstrate immune responses and rejection of male organs or skin following transplantation mediated by female animals (2X chromosomes) against male cells (X and Y chromosome). It is not possible to show responses against female antigens by male animals because the male animals have one X chromosome, and so are tolerant to all antigens encoded on it (Fig. 21.6).