- 8.1 Introduction
- 8.2 Histocompatibility genetics in humans
- 8.3 Renal transplantation
- 8.3.1 Selection of recipient and donor
- 8.3.2 Post-transplantation period
- 8.3.3 Clinical rejection
- 8.3.4 Immunopathology of rejection (the allograft response)
- 8.3.5 Graft survival
- 8.3.6 Complications
- 8.3.1 Selection of recipient and donor
- 8.4 Other types of transplantation
- 8.4.1 Liver transplantation
- 8.4.2 Heart transplantation
- 8.4.3 Lung transplantation
- 8.4.4 Pancreatic transplantation
- 8.4.5 Skin grafting
- 8.4.6 Corneal grafting
- 8.4.1 Liver transplantation
- 8.5 Haematopoietic stem cell transplantation
- 8.5.1 Indications and selection of patients
- 8.5.2 Management of the patient
- 8.5.3 Complications of allogeneic HSCT and their prevention: graft-versus-host disease and infection
- 8.5.4 Results
- 8.5.1 Indications and selection of patients
 Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
8.1 Introduction
Transplantation of living cells, tissues or organs is well established as a routine practice. Cells (e.g. red blood cells, stem cells), tissues (e.g. skin grafting in extensively burned patients) or whole organs (such as kidney, heart, lung, pancreas or liver) may be successfully transferred between genetically dissimilar individuals (allogeneic grafting). The outcome depends on the degree of ‘matching’ of the relevant transplantation antigens of the two individuals and successful therapeutic immunosuppressive measures to prevent rejection. In contrast, grafting of an individual’s tissue from one part of the body to another (autologous grafting) is always successful, provided there are no procedural setbacks.
8.2 Histocompatibility genetics in humans
The surfaces of all human cells express a series of molecules that are recognized by other individuals as foreign antigens. Some antigens, such as those of the rhesus blood group, are not relevant to the successful transplantation of human organs. In contrast, the ABO blood group system on red blood cells and the human leucocyte antigens (HLAs) on lymphocytes and other tissues are extremely important in blood transfusion and organ transplantation. HLA antigens are also called ‘histocompatibility antigens’ since they play a crucial role in determining survival of transplanted organs. They are encoded in humans by a segment of chromosome 6 known as the major histocompatibility complex (MHC) (see Chapter 1). Additional antigenic systems (minor histocompatibility systems) play only a minor role in transplantation and are largely ignored in clinical practice.
At least six HLA loci are recognized (Fig. 8.1). The HLA-A and HLA-B loci were the first to be defined and these code for a large number of antigens (see Chapter 1). Together with the HLA-C locus, these MHC class I genes code for products of similar biochemical structure that serve similar functions (see Chapter 1). They are detectable on all nucleated cells in the body. The methods of tissue typing, i.e. the detection of HLA antigens, is described in Chapter 19.
Fig. 8.1 Major histocompatibility complex on chromosome 6; class III antigens are complement components.
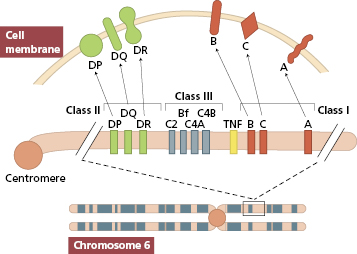
In contrast to antigens of the HLA-ABC loci, HLA-D loci antigens are restricted to B lymphocytes, macrophages, epidermal cells and sperm. The antigens of the D loci differ from MHC class I antigens in their chemical structure and interactions with immune cell populations; they are called MHC class II antigens (see Chapter 1).
In renal transplantation, matching for the MHC class II antigens is more important than MHC class I antigen compatibility in determining graft survival. Matching for the ABO blood group system is also important; naturally occurring anti-A and anti-B antibodies can lead to hyperacute rejection of ABO-incompatible kidneys, since A and B antigens are expressed on endothelium.
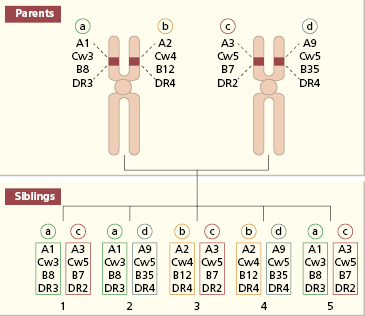
In bone marrow transplantation, a complete match of chromosome 6 gives the best survival; this is provided by an autologous graft, an identical twin or HLA-identical sibling. Mismatched bone marrow invariably induces graft-versus-host disease (GVHD) with reduced survival of the graft (see section 8.5.3). The proximity of the HLA loci means that their antigenic products tend to be inherited together as an ‘HLA-haplotype’. Because one haplotype is inherited from each parent, there is a one-in-four chance that two siblings will possess identical pairs of haplotypes (Fig. 8.2).
Fig. 8.2 Inheritance of HLA haplotypes in a family. Siblings 1 and 5 are HLA identical. Haplotypes are denoted as a, b, c, d.
8.3 Renal transplantation
Kidney transplantation is now widely available for the treatment of end-stage chronic renal failure. Haemodialysis and chronic ambulatory peritoneal dialysis have enabled patients to come to transplantation in a state fit to withstand major surgery. The survival figures for transplanted kidneys have shown progressive improvement over the decades because of better immunosuppression, with consequent reduction in mortality of patients. A kidney transplant is the treatment of choice for most patients in end-stage renal failure.
8.3.1 Selection of recipient and donor
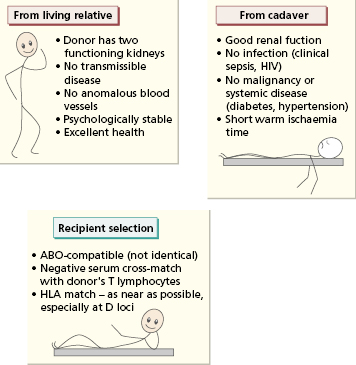
Criteria for selection of patients for renal transplantation vary between centres. Extreme old age, severe sepsis, a severe bleeding tendency or any other contraindications to high-dose steroids make a patient unacceptable as a potential recipient. Once selected, the patient has to wait for a suitably matched kidney to become available. Two types of donor kidneys are used: those from cadavers and those from living related donors.
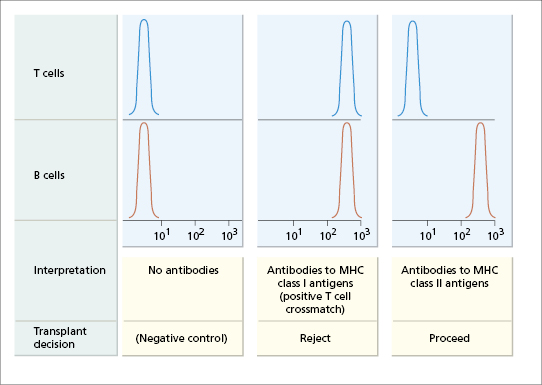
The selection of cadaveric kidneys is rigorous (Fig. 8.3). In addition to the kidney, the spleen is also removed and disrupted, and the resulting lymphocyte suspension is used to detect MHC class I and class II antigens by molecular methods (see Chapter 19). Only patients with an ABO blood group compatible with the kidney donor are considered suitable recipients; as in blood transfusion, a group O kidney can be transplanted to any recipient. Knowing the ABO blood group and HLA type of a cadaver kidney, the national register of potential recipients is searched to find an ABO-compatible patient who matches the donor at as many loci as possible. Having selected the recipient, the recipient’s serum is then cross-matched against the donor’s lymphocytes (Fig. 8.4). If the patient has cytotoxic antibodies to donor class I antigens (positive T-cell cross-match), then the kidney is unsuitable for that recipient.
Fig. 8.4 Schematic representation of detection of cytotoxic antibodies in renal transplant recipients by flow cytometric cross-matching. Fluorescence intensity is depicted on the horizontal axis and the number of donor cells on the vertical axis (see Chapter 19 for discussion of flow cytometry). MHC class II antigens are expressed on B cells but not on T cells; MHC class I antigens are expressed on both.
Relatives who are anxious to donate a live donor kidney must be screened clinically and psychologically (see Fig. 8.3), and ABO and HLA typed so that the most suitable donor can be chosen. As discussed earlier, there is a one-in-four chance that a sibling will have the identical HLA haplotype (see Fig. 8.2). Where a compatible donor cannot be found, an attempt is made to choose someone with the least disparity at the HLA-A and HLA-DR loci, as these are the most important loci governing rejection of the graft.
Once the kidneys have been removed from either donor type, they are perfused mechanically with physiological fluids. Provided that cooling is begun within 30 min of cessation of the renal blood supply (warm ischaemia time), these kidneys have an excellent chance of functioning in the selected recipient. The duration of the perfusion (cold ischaemia time) should be less than 48 h. The transplanted kidney is usually sited in the iliac fossa. Great care is taken with the vascular anastomoses and ureteric implantation. Once the vascular anastomoses are complete, the graft often starts to function immediately.
8.3.2 Post-transplantation period
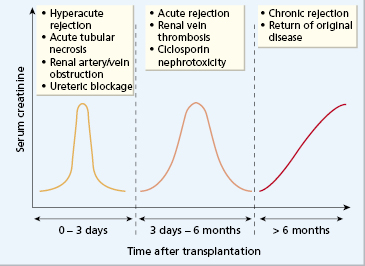
In the post-transplantation period, the graft and the patient must both be monitored. There are several reasons why renal function may deteriorate immediately after surgery (Fig. 8.5). Acute tubular necrosis can occur due to low blood pressure in either the recipient or the donor. If this happens, the recipient can be dialysed until renal function recovers; this rarely takes longer than 3–4 weeks. Alternatively, poor renal function may indicate hyperacute rejection, urinary obstruction, which must be relieved surgically, or a vascular event; arterial perfusion and venous drainage can be assessed by duplex ultrasonography.
It is crucial to distinguish rejection from infection, since the treatment for a bacterial infection is an antibiotic, not an increase in immunosuppressive therapy! Regular core renal biopsies may be performed, but the procedure can damage the kidney and should not be done more than once every few weeks. Detection of rejection by frequent percutaneous fine-needle aspiration is much less traumatic, gives quick results and allows identification of infiltrating monocytes and increased expression of MHC class II molecules on renal tubular cells in the cortex – features of rejection (Case 8.1).
 Case 8.1 Acute rejection
Case 8.1 Acute rejectionAn 18-year-old student with end-stage renal failure due to chronic glomerulonephritis was given a cadaveric kidney transplant. He had been on maintenance haemodialysis for 2 months, and on antihypertensive therapy for several years. His major blood group was A and his tissue type was HLA-A1, -A9, -B8, -B40, -Cw1, -Cw3, -DR3, -DR7. The donor kidney was also blood group A and was matched for one HLA-DR antigen and four of six HLA-ABC antigens. He was given triple immunosuppressive therapy with ciclosporin, azathioprine and prednisolone.
He passed 5 L of urine on the second post-operative day and his urea and creatinine fell appreciably. However, on the seventh postoperative day, his graft became slightly tender, his serum creatinine increased and he had a mild pyrexia (37.8°C). A clinical diagnosis of acute rejection was confirmed by a finding of lymphocytic infiltration of the renal cortex on fine-needle aspiration. A 3-day course of intravenous methylprednisolone was started. Twenty-four hours later his creatinine had fallen and urine volume increased.
Subsequently, the patient had similar rejection episodes 5 and 7 weeks post-operatively. Both were treated with intravenous corticosteroids, and he has since remained well for over 3 years. Ciclosporin A was discontinued after 9 months but he still takes a daily maintenance dose of immunosuppressive drugs, namely 5 mg prednisolone and 50 mg azathioprine.
Immunosuppressive therapy (Table 8.1) is intended to prevent graft rejection and remains an essential component of management. Immunosuppressive drugs are fully discussed in Chapter 7.
Table 8.1 Drugs used as antirejection therapy in renal transplantation
| Prevention of graft rejection | Treatment of acute rejection |
|---|---|
| Prednisolone | Methylprednisolone |
| Ciclosporin | Antithymocyte globulin |
| Tacrolimus (FK506) | |
| Mycophenolate mofetil | |
| Monoclonal antibodies against the αchain of the IL-2 receptor (CD25) on T cells or CD52 (CAMPATH-1) |
OKT3, Anti-CD3 monoclonal antibody.
The introduction of calcineurin inhibitors – ciclosporin and later tacrolimus – in the mid-1980s significantly improved short-term renal graft survival by lowering acute rejection rates but chronic nephrotoxicity was a cause of declining transplant function. Hence, various combinations are used to avoid such nephrotoxicity, including monoclonal antibodies, mycophenolate mofetil or rapamycin. Most protocols reserve lymphocyte-depleting monoclonal antibodies for rejection therapy, and use steroids and ciclosporin or tacrolimus for prophylaxis. Conversion from a calcineurin inhibitor-based regimen to another long-term agent such as rapamycin is undertaken between 3 and 6 months post transplant. Immunosuppression in children undergoing kidney transplantation is more difficult and mycophenolate mofetil-based regimens with low-dose calcineurin inhibitor therapy and corticosteroids are preferable. In patients with chronic allograft dysfunction and over-immunosuppression leading to recurrent infections, mycophenolate mofetil and corticosteroids are more appropriate. Results show that for regimens consisting of a calcineurin inhibitor combined with mycophenolate mofetil and corticosteroids, >90% of all renal grafts are functioning after 1 year and with 77% and 56% at 5- and 10-years respectively. Additionally, in some centres monoclonal antibody therapy is included in the induction stage to reduce the risk of acute rejection. The most commonly used are Basiliximab or Daclizumab (against the α-chain of the IL-2 receptor on T cells (CD25) and Alemtuzumab (CAMPATH-1H – anti CD52) (see Chapter 7).
8.3.3 Clinical rejection
Rejection may occur at any time following transplantation. The classification of rejection into early, short term and long term reflects the differing underlying mechanisms (Fig. 8.5).
Hyperacute rejection may occur a few minutes to hours following revascularization of the graft. It is due to preformed circulating cytotoxic antibodies in the recipient that react with MHC class I antigens in the donor kidney. A similar picture is seen if an ABO-incompatible kidney is inadvertently used. Activation of complement results in an influx of polymorphonuclear leucocytes, platelet aggregation, obstruction of the blood vessels and ischaemia. The patient may be pyrexial with a blood leucocytosis. Histologically, the microvasculature becomes plugged with leucocytes and platelets, resulting in infarction. The kidney swells dramatically and is tender. Red cells and desquamated tubular cells are often found in the urine. Renal function, which normally starts within minutes of revascularization of the graft, declines; oliguria or anuria follows. There is no successful therapy and the kidney must be removed immediately. With improved cross-match techniques (see Fig. 8.4), hyperacute rejection has become very uncommon.
Acute rejection occurs within a few weeks or months following transplantation. Early diagnosis is important because prompt treatment with intravenous methyl prednisolone and/or anti-CD3/ATG reverses renal damage. Clinical features may be masked by ciclosporin; a rising serum creatinine and a mild fever may be the only signs. It is important to exclude urinary obstruction or perirenal collections of urine, blood or pus. Histologically, there is a mononuclear infiltrate in cellular rejection with necrosis of arterial walls; after successful treatment, the inflammatory infiltrate clears (Fig. 8.6). Acute rejection is associated with increased expression of MHC class I and class II antigens in inflamed grafts, and with early infiltration of CD8+ T lymphocytes. Fine-needle aspiration helps to distinguish rejection from ciclosporin toxicity and acute humoral rejection with granulocytes and complement deposition.