Diabetic peripheral neuropathy
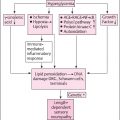
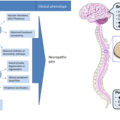
Diabetic peripheral neuropathy (DPN) affects about 50% of the diabetes population and an effective treatment has eluded investigators for decades in spite of repeated successful discoveries in animal models. Human and animal studies have widely demonstrated that the etiology of DPN is complex with multiple mechanisms contributing to vascular and nerve dysfunction, which are believed to be the primary factors responsible for sensory and motor nerve impairment . Hyperglycemia has long been thought to be the primary cause of peripheral neuropathy in patients with type 1 diabetes but numerous studies have shown that good glycemic control is not sufficient to significantly alter the course of this disease in patients with type 2 diabetes . This scenario is further complicated by the realization that both animal and human subjects with impaired glucose tolerance and insulin resistance develop forms of sensory neuropathy . There are many possible reasons for the lack of success of translation of effective treatments in animal models for DPN to human subjects. This includes the possibility that diabetic animals do not adequately model human DPN, poor design of clinical trials such as duration of disease, selected primary endpoints and length of treatment compromises the overall outcome of the study, large placebo effect commonly seen in human trials for DPN for some endpoints masks any true benefit, and singular interventions targeting only one potential mechanism for a disease with multiple etiologies. Whatever the reasons for the failure of past clinical trials there remains a dire need to find a treatment that will reduce the impact of DPN on patients, family, and society. In this chapter, we will examine evidence that the combination of fish oil [enriched in the omega-3 polyunsaturated fatty acids (PUFAs); eicosapentaenoic acid (20:5, EPA), and docosahexaenoic acid (22:6, DHA)] and salsalate may be an effective treatment for DPN and worthy of advancing to a clinical trial.
Omega-3 polyunsaturated fatty acid; eicosapentaenoic acid and docosahexaenoic acid
Omega-6 and omega-3 PUFA are essential fatty acids with multiple double bonds, the first of which is located at the sixth or third carbon from the methyl end of the molecule, respectively . Mammals lack enzymes that insert a double bond in the omega-6 and omega-3 position of fatty acids; therefore linoleic acid (18:2, omega-6 PUFA), γ-linolenic acid (18:3, omega-6 PUFA), and α-linolenic acid (18:3, omega-3 PUFA), as well as some of their elongation products including EPA and DHA, are deemed to be essential dietary nutrients . The omega-6 fatty acids are derived from the diet primarily as linoleic acid and are elongated in vivo to γ-linolenic acid and arachidonic acid (AA) . Most eicosanoids derived from AA are proinflammatory, whereas metabolites derived from omega-3 PUFA have antiinflammatory properties. The balance of omega-6 to omega-3 fatty acid consumption can affect systemic inflammatory processes and immune activity . The ratio of consumed omega-6 to omega-3 fatty acids in the Western diet is approximately 15 to 1, whereas a ratio of 4 to 1 is considered optimal . Plants, seeds, nuts and their oils are a good source for α-linolenic acid, but fish oils are the primary source for the long-chain omega-3 PUFA such as EPA and DHA. In mammals, EPA and DHA can also be produced in small amounts from α-linolenic acid. This synthesis is thought to occur through a series of desaturation (involving Δ6, Δ5, and Δ4 desaturases) and elongation steps ( Fig. 20.1 ) . However, it is now considered more likely that DHA is biosynthesized via a 24 carbon intermediate fatty acid followed by beta-oxidation in peroxisomes. In this pathway, EPA is twice elongated, yielding 24:5 ω-3, then desaturated to 24:6 ω-3, then shortened to DHA via beta-oxidation. This pathway is known as Sprecher’s shunt .
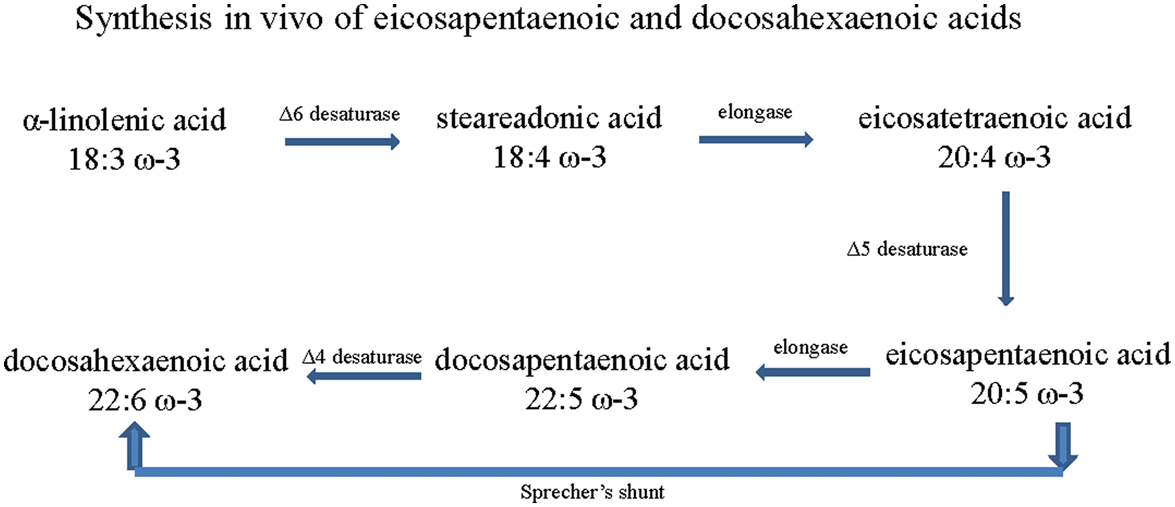
In subjects with diabetes, the formation of EPA and DHA via α-linolenic acid is impaired . Furthermore, diabetes-induced changes in Δ-5 desaturase activity causes an increase in the omega-6 to omega-3 fatty acid ratio, further promoting an inflammatory state that contributes to the pathologies associated with obesity and diabetes as well exacerbation of DPN . Thus, increasing the circulatory levels of EPA and DHA, and favorably altering the omega-6 to omega-3 fatty acid ratio could be an effective therapy for treating DPN. The most effective approach to increasing circulating levels of EPA and DHA is through the diet and consuming foods enriched in these lipids such as oily fish or by taking supplements such as fish oil capsules. Different forms of algae have also been isolated and commercially grown that produce primarily EPA or DHA but studies of the impact of these selectively enriched omega-3 PUFA oils on health outcomes are lacking. However, there is great interest in pursuing studies of these oils for environmental reasons. Few side effects have been associated with taking the recommended doses of fish oil capsules containing omega-3 PUFA. The minor side effects that have been reported include loose stools, stomach upset, and belching (that includes a “fish stick” like the taste). However, consumption of omega-3 PUFA in isolated cases may raise the risk of bleeding and this can be potentially serious for subjects with bleeding disorders or taking blood-thinning medications. Thus, subjects with a known risk for bleeding disorders are consulted to seek the advice of their physician before taking fish oil-containing capsules.
Omega-3 polyunsaturated fatty acid and cardiovascular health
There is significant rationale to pursue fish oil and salsalate as a potential treatment of DPN. Cardiovascular disease has been the primary disorder to be evaluated for treatment by omega-3 PUFA . However; additional studies suggest potential benefits of omega-3 PUFA consumption on other chronic diseases including chronic inflammatory and autoimmune diseases, stroke, muscle atrophy, and neurodegenerative disease . Nonetheless, the consumption of omega-3 PUFA remains low in the Western diet due to historically increased consumption of omega-6 PUFA.
There has been a mixed response and sometimes controversial regarding the potential benefits of omega-3 PUFA on cardiovascular disease . However, recent literature is describing that beneficial effects of omega-3 PUFA supplementation on cardiovascular disease risk are apparent when subjects achieve higher blood levels of omega-3 fatty acids and when the omega-3 index is in a healthy range . The omega-3 index is defined as the sum of EPA and DHA as a percentage of total fatty acids in red blood cells . This raises an important issue because most of the trials that have been conducted examining the effect of omega-3 PUFA on cardiovascular disease did not include blood levels of fatty acids in their outcome measures. This flaw in study design creates a doubt in the outcome of these studies when negative since variability in subjects enrolled in these trials achieving a therapeutic blood level of omega-3 PUFA can be influenced by many factors including, genetic variability, noncompliance or low dosing as well as by gender, age, body weight, and physical activity . For example, in the recent ASCEND study, 15,480 subjects with diabetes received 1 g capsules daily containing omega-3 fatty acids or placebo. In this study, the primary endpoint was a first serious vascular event. After a mean follow-up period of 7.4 years, the study found no significant difference in the risk of serious vascular events between those assigned to receive omega-3 fatty acids or placebo . In contrast, the REDUCE-IT trial enrolled 8179 subjects that included subjects with diabetes to receive a 4 g daily dose of icosapent ethyl, a highly enriched form of an ester derivative of EPA, or placebo. After a follow-up period of 4.9 years, icosapent ethyl was found to have a statistical benefit on reducing ischemic events in subjects with hypertriglyceridemia . In August of 2019, the American Heart Association released a Science Advisory based on examination of the REDUCE-IT trial and other studies using “pharmacological doses” of omega-3 fatty acids (>3 g/day total EPA+DHA) stating that omega-3 fatty acids (EPA+DHA or EPA-only) are an effective and safe option for reducing triglycerides as monotherapy or as an adjunct to other lipid-lowering agents .
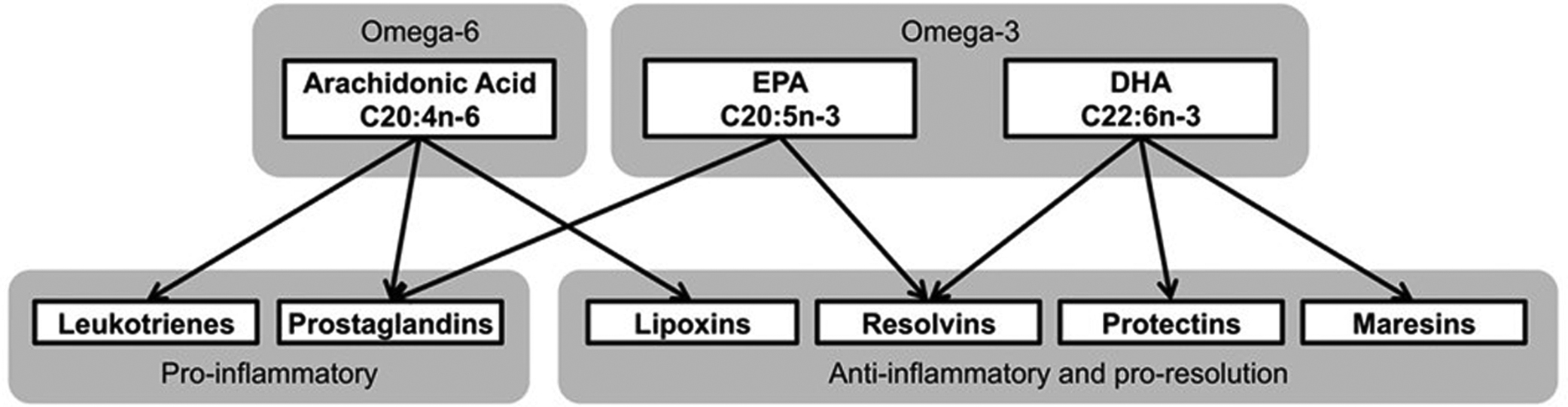
Was the apparent success of the REDUCE-IT trial due to increased dosing of the omega-3 PUFA derivative, study design, or other factors? This question is difficult to answer because neither trial analyzed circulation levels of omega-3 PUFA or their metabolites before and after treatment. The failure to measure levels of omega-3 fatty acids in the blood of subjects enrolled in trials examining the potential benefits of fish oils is a serious error. Individual variability could be a limiting factor in the success or failure of omega-3 PUFA therapy. This is highlighted by a study of a Japanese population that reported 4 g of fish oil per day failed to achieve the target EPA/AA ratio in the blood in 16% of the enrolled subjects . In our animal studies, to be summarized in this chapter, fish oil treatment variability in the accumulation of omega-3 fatty acids in blood and tissues is not an issue. Animals used for preclinical studies are genetically similar and gender, age, body weight, and activity are all usually well matched. However, this is not the case in studies of human subjects and it is important that each subject enrolled in future studies be evaluated for changes in omega-3 fatty acid levels as an outcome measure. Moreover, since the metabolites of omega-3 PUFA such as resolvins and neuroprotectin-1 (NPD-1) are the potential mediators of the beneficial effects of fish oil the circulating levels of these compounds should also be determined ( Fig. 20.2 ) . However, there is no information from clinical trials using omega-3 PUFA on the levels of these antiinflammatory metabolites. Furthermore, it is unknown what variability may exist for the accumulation of omega-3 PUFA in circulation (omega-3 index) or in the formation of their metabolites due to diabetes. Therefore future studies of the potential benefits of omega-3 PUFA as disease–modifying therapy should also include matched control subjects in order to determine what variability may exist in the metabolism of these fatty acids within the human population and disease being studied. For instance, it is unknown if diabetes in the absence or presence of DPN will affect the omega-3 index or profile of production of the antiinflammatory mediators of omega-3 PUFA. Since the antiinflammatory metabolites of omega-3 PUFAs have neuroprotective properties, we hypothesize that a therapy leading to increasing their production in vivo may be a potential disease-modifying therapeutic approach for DPN.
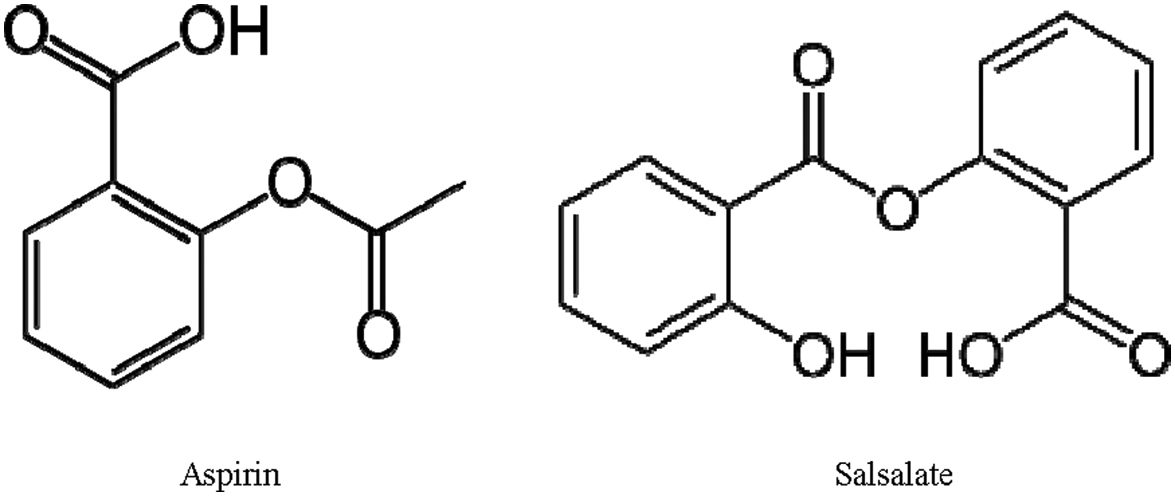
Aspirin has been reported to indirectly increase the production of resolvins , although the long-term use of an effective dose of aspirin is prohibitive due to multiple unfavorable side effects. However, salsalate, a prodrug of salicylate, is well tolerated and considered safe for long-term clinical use ( Fig. 20.3 ) . Both aspirin and salsalate are rapidly broken down to salicylate in vivo . In high-fat-fed mice salsalate activates brown adipose tissue and improves glucose tolerance . In GK rats salsalate increased levels of adiponectin and decreased adipose-tissue-derived inflammation . In patients with diabetes, salsalate alleviated insulin resistance, improved metabolic control, and improved the lipid, lipoprotein, and apoprotein profile of insulin-resistant individuals who were overweight or obese . In our preclinical studies salsalate alone provided some improvement in DPN (to be discussed further below); however, salsalate in combination with fish oil increased the production of resolvins and had greater benefit toward vascular and neural endpoints associated with DPN than fish oil alone .
Omega-3 polyunsaturated fatty acid and vascular dysfunction
Our studies as well as others have shown that vascular dysfunction is a contributing factor to the development and progression of DPN . Thus, any potential treatment for DPN should also improve and/or protect vascular function. A number of studies in animal models of obesity and diabetes including our own have demonstrated that treating rodents with omega-3 PUFA improves vascular function. Our studies with type 2 diabetic rats treated with a diet enriched with menhaden oil have demonstrated significant improvement in vascular reactivity of epineurial arterioles, blood vessels that provide circulation to the sciatic nerve, to acetylcholine and calcitonin gene-related peptide . Other animal studies have reported that in type 2 diabetic rats a diet enriched in DHA prevented diabetic retinopathy by inhibiting retinal vascular damage . In type 2 diabetic Otsuka Long-Evans Tokushima fatty rats EPA ameliorated endothelial dysfunction in mesenteric arteries by correcting the imbalance between endothelium-derived factors in part by inhibiting extracellular signal-regulated kinase, decreasing nuclear factor-κB and reducing cyclooxygenase-2 expression . Farooq et al. in studies using “old rats” showed that treating these rats with an omega-3 formulation of 6:1 ratio of EPA and DHA improved endothelium-dependent relaxations . An effect they attributed to prevention of the upregulation of the local angiotensin system and reduction of reactive oxygen species. We have also demonstrated that blocking the angiotensin system with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker improved vascular relaxation by epineurial arterioles as well as peripheral neuropathy in both type 1 and type 2 diabetic rats . Working with cultured vascular endothelial cells, Sakai et al. demonstrated that fish oil-derived omega-3 PUFA prevented oxidative stress-induced DNA damage . In ob/ob mice a diet enriched in omega-3 PUFA significantly improved insulin resistance and vascular reactivity of the aorta . In KKAy mice, a model of type 2 diabetes, EPA ethyl ester was shown to improve endothelial dysfunction and vascular reactivity to acetylcholine in aortic rings . In human subjects, it has been demonstrated that omega-3 PUFA consumption improved small peripheral artery function in patients at intermediate-high cardiovascular risk . In addition, it has been shown that increasing the EPA to AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia . In summary, multiple studies in different animal models of diabetes as well as human subjects have demonstrated that omega-3 PUFA derived from fish oil protects endothelial cells from oxidative stress and vascular reactivity by different vascular beds.
Omega-3 polyunsaturated fatty acid and peripheral neuropathy related to obesity and insulin resistance
Studies in animal models of prediabetes and humans with impaired glucose tolerance and insulin resistance have been shown to develop forms of sensory neuropathy . We have shown that rats fed a high-fat diet progressively develop impaired glucose utilization and slowing of sensory nerve conduction velocity, loss of sensory nerves in the skin and cornea and decreased sensitivity of intraepidermal nerve fibers to a thermal stimulus and corneal nerves to Cochet–Bonnet filament esthesiometer . All these deficits were reversed by enriching their high-fat diet with menhaden oil . Obesity as described in our high-fat-fed rat model is often associated with low-grade inflammation due to overproduction of inflammatory mediators by adipose tissue . Increased consumption of omega-3 PUFA or exogenous administration of resolvins, antiinflammatory and proresolving mediators endogenously generated by EPA (E series resolvins) and DHA (D series resolvins), reduce the inflammatory condition ( Fig. 20.4 ) .
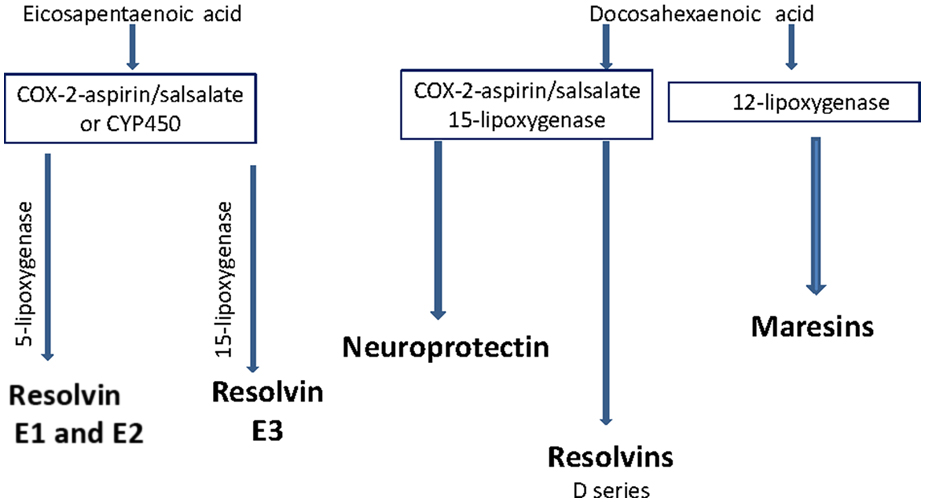
In obesity, the increased inflammatory state and increased levels of leptin and reduced levels of adiponectin are corrected with increased consumption of omega-3 PUFA or exogenous treatment with resolvins . In adipose tissue, it appears that increased consumption of EPA and DHA lowers lipogenesis, increases lipolysis, and inhibits the nuclear factor-κB pathway thereby decreasing inflammation and oxidative stress . Preclinical studies have shown that increased consumption of omega-3 PUFA or exogenous treatment with resolvins improves insulin resistance in animal models of obesity and diabetes . Studies conducted with obese and type 2 diabetic human subjects have also shown that increased consumption of long-chain omega-3 PUFA improves insulin sensitivity . Several studies have even shown that individuals with higher serum levels of omega-3 PUFA and those that consume lean fish are at a reduced risk of developing type 2 diabetes . However, this remains controversial since an earlier study found no evidence that higher consumption of long-chain omega-3 PUFA and fish reduces the risk of type 2 diabetes . In summary, preclinical studies and studies with human subjects have demonstrated that increasing the consumption of omega-3 PUFA can improve many of the pathogenic characteristics associated with obesity.
Omega-3 polyunsaturated fatty acids and diabetic peripheral neuropathy
Omega-3 PUFA have long been implicated in the development and continued maintenance of healthy nerves and may be beneficial for promoting peripheral nerve restoration . Our body of work over the last 8 years have demonstrated that enriching the diet of chronic diabetic mice and rats with menhaden (fish) oil, enriched in EPA and DHA, can not only slow progression of DPN as determined by protection of motor and sensory nerve conduction velocity and prevention of thermal hypoalgesia but can stimulate reversal of neurovascular and neuropathic deficits and repopulation of sensory nerves in the skin and cornea . These latter data is of potential interest from a clinical and translational point. Historically intervention trials for treatment of DPN have depended on measurement of large fiber function as the primary endpoint and outcomes from these trials have been disappointing. More recent studies have suggested that damage to small sensory nerve fibers in the skin and cornea precede large nerve fiber damage and may be a better early marker for DPN . Clinical trials focusing on structural and functional changes of these nerves as a primary endpoint for the treatment of DPN are needed. In this regard a proof-of-concept study focusing on the effect of 12 months of omega-3 PUFA supplementation sought to determine whether omega-3 PUFA could stop the known progression of diabetic sensorimotor polyneuropathy in type 2 diabetes subjects by measuring changes in corneal nerves and measures of small and large fiber function . In this study subjects were treated for 12 months with a 10 mL dose of seal oil containing 2.33 g of omega-3 PUFA (0.75 g EPA, 0.56 g docosapentaenoic acid, and 1.02 g DHA) daily. The primary outcome measure was the 1-year change in corneal fiber length measured by in vivo corneal confocal microscopy, with sensory and nerve conduction measures as secondary outcomes. Unfortunately the authors of this study did not examine the change in omega-3 PUFA in serum following the study or the effect of treatment on the omega-3 index or change in omega-6 to omega-3 PUFA ratio in serum. The primary outcome measure showed a 29% significant increase in corneal nerve fiber length. There was no significant change in the secondary outcome measures. The change in corneal fiber length in these type 1 diabetic subjects was comparative to the increase in corneal fiber length we have shown to occur in type 1 diabetic rats supplemented with menhaden oil in their diet . In another recent study omega-3 PUFA supplementation of type 1 diabetes subjects was used to determine the effect on vascular health, glycemic control and metabolic parameters . In this placebo controlled study type 1 diabetes subjects were treated for 6 months with 3.3 g/day encapsulated omega-3 PUFA. Following treatment for 6 months the omega-3 PUFA index significantly increased from 4.9%±0.9% to 8.3%±1.5%. However, in this study enrichment in omega-3 PUFA did not improve vascular health, glucose homeostasis, or metabolic parameters. The authors concluded that their preliminary randomized controlled trial does not support the use of therapeutic omega-3 PUFA supplementation in the treatment and management of type 1 diabetes. It should be noted that this trial was of short duration, did not examine any endpoints related to DPN or other diabetes complications and did not measure circulating levels of omega-3 PUFA metabolites. In a much older study subjects with type 2 diabetes were treated for 49 weeks with 1.8 g/day of ethyl ester of EPA . A similar EPA formulation was used in the REDUCE-IT trial that was found to improve after 4.9 years of treatment cardiovascular disease and reduce serum triglyceride levels . In this study, the authors also noted a decrease in serum triglyceride levels in their treated subjects as well as an improvement in vascular function and clinical symptoms related to sensory nerve function and vibration perception threshold of the lower extremities . Clearly more clinical studies are needed to determine whether omega-3 PUFA may be an effective therapy for DPN. These trials need to be of sufficient duration and use a therapeutic dose of omega-3 PUFA that not only increases blood levels of omega-3 PUFA but also their metabolites. The importance of measuring circulating levels of omega-3 PUFA metabolites is supported by our studies that have shown that treating type 1 and type 2 diabetic mice with daily injections of resolvin D1 improved DPN .
Since the antiinflammatory metabolites of omega-3 PUFAs have neuroprotective properties, it seems reasonable to hypothesize that a therapy leading to increasing their production in vivo may be a potential disease-modifying therapeutic approach for DPN. Aspirin has been reported to indirectly increase the production of resolvins , although the long-term use of an effective dose of aspirin is prohibitive due to multiple unfavorable side effects (see Fig. 20.4 ). However, salsalate, a prodrug of salicylate, is well tolerated and considered safe for long-term clinical use . In phase 3 clinical trial performed in patients with type 2 diabetes salsalate was shown to decrease inflammatory mediators, lower fasting blood glucose and HbA 1 C levels, and improve lipid profiles . In our preclinical studies salsalate alone provided minimal improvement in DPN; however, salsalate in combination with fish oil increased the production of resolvins and had greater benefit toward DPN than fish oil alone (see Fig. 20.4 ) .
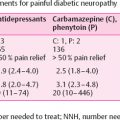
In our studies, we have attributed the beneficial effects of menhaden oil on diabetic peripheral neuropathy to the reduction of oxidative and inflammatory stress and the neuroprotective properties of resolvins and neuroprotectin . Other laboratories have reported similar results. Gerbi et al. reported that fish oil supplementation prevents diabetes-induced slowing of nerve conduction velocity, neuroanatomical changes in rats and improves sciatic nerve Na,K-ATPase activity . Julu reported that essential fatty acids that included EPA prevented slowing of nerve conduction velocity in streptozotocin-induced diabetic rats . Some recent studies have suggested that treatment with fish oils may also be beneficial in preventing painful diabetic neuropathy. Heng et al. reported that dietary supplementation of DHA inhibited mechanical allodynia and thermal hyperalgesia by decreasing the excitability of dorsal root ganglions . Li et al. reported that supplementing the diet of diabetic rats with fish oil prevented mechanical allodynia and thermal hyperalgesia by blocking nuclear factor-κB-mediated inflammatory pathways . Wagner et al. have shown in a murine diabetic model that DHA is metabolized into epoxydocosapentanoic acids via cytochrome P450 enzymes and these metabolites are analgesic against chronic pain and more efficacious than opioids . Another study demonstrated that local administration of DHA reverted hyperalgesia and allodynia in diabetic rats with the results indicating that the antihyperalgesic effect of DHA involves indirect activation of µ and δ receptors .
In conclusion, I have provided a condensed overview of some of the beneficial effects attributed to omega-3 PUFA with the primary focus on diabetic peripheral neuropathy. I have provided evidence from our studies that the metabolites of omega-3 PUFA, resolvins and neuroprotectin, are likely responsible for the reported benefits of these lipids. Biologically active concentrations of these antiinflammatory lipid mediators in serum can be achieved following oral omega-3 PUFA supplementation . Moreover, preclinical studies from my laboratory have demonstrated that salsalate can substitute for aspirin and when combined with fish oil increases the circulatory levels of resolvin and the efficacy of fish oil toward diabetic peripheral neuropathy. Given the availability of omega-3 PUFA as an over the counter supplement and their safety profile, after multiple studies of improvement in cardiovascular health, enriching the daily intake of these lipids, unless the individual has some form of a bleeding disorder, seems like a reasonable approach to improve the health of subjects with diabetes. Given the body of evidence of the many potential benefits of increased consumption of omega-3 PUFA it seems that it is time to seriously consider human clinical trials using fish oil and salsalate for the treatment of diabetic peripheral neuropathy.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree