Female
Male
Hypertensive disorders of pregnancy
Coronary artery dissection of pregnancy
“hot flashes” of menopause
Pulmonary hypertension
Raynaud’s disease
Microvascular angina
Migraine
Postural orthostatic tachycardia syndrome (POTS)
Heart failure with preserved ejection fraction (HFpEF)
Apical Ballooning (Tako-Tsubo)
Erectile dysfunction
Systemic hypertension
Myocarditis
Phenotypic Expression of Sex Differences in Cardiovascular Disease
Hypertension
Although men may present with systemic hypertension at an earlier age than women, the incidence of pulmonary hypertension is greater in women than men [29–31]. Alterations in sympathetic activity affects vascular tone and subsequently arterial pressure and blood flow to end-organs such as the heart, brain, liver, and skeletal muscle [9]. As discussed above, regulation of adrenergic neurotransmission is affected by the presence of both the sex chromosomes and sex steroid hormones. In men, increases in resting sympathetic vasoconstrictor activity increases total peripheral vascular resistance [32] which may contribute to the manifestation of hypertension in men at younger ages than in women [33]. However, in young women, increases in sympathetic nerve activity do not cause a linear increase in total peripheral resistance or cardiac output [34]. Additional evidence demonstrating effects of hormones on sympathetic-mediated changes in blood flow are observed in changes in skin blood flow during a woman’s menstrual cycle [35, 36]. These changes suggest that female sex steroids may modulate the response of the cutaneous circulation of the head, limb, and trunks that are controlled by adrenergic and cholinergic neurons that lead to vasoconstriction and vasodilatation, retrospectively [37]. Relationships between sympathetic nerve activation and total peripheral resistance are just beginning to be explored in menopausal women [38], but the peripheral and central factors controlling mechanisms of vasomotor disturbances (hot flashes/flushes) of menopause remain unknown.
Other examples of cardiovascular disease having a female predominance most likely reflecting differences in autonomic function are vasomotor disorders such as Raynaud’s and postural orthostatic tachycardia syndrome (POTS) [9].
Stroke incidence, on the other hand, demonstrates a male predominance. For men, stroke incidence rates have been reported at 20.7 per 10,000, whereas for women rates are 9.6 per 10,000 [39]. Systemic hypertension is a major risk factor for stroke. Therefore, as the rates of hypertension in women go up after menopause, so too does this chance of stroke. Treating systolic hypertension can significantly lower the incidence of stroke [40], including hemorrhagic, ischemic, and lacunar stroke.
Sex differences in underlying physiological mechanisms will affect not only manifestations of disease (symptoms) but also response to treatment. For example, presentation of symptoms for myocardial infarction may differ in men and women with more nonspecific or abdominal-related symptoms seen in women and treatments for hypertension while effective in women may not always result in reduction of blood pressure in women to treatment targets [41].
Heart Failure with Preserved Ejection Fraction
Heart failure can manifest with depressed or preserved ejection fraction. In women, it is more common to find heart failure with preserved ejection fraction (HFpEF). The major risk factors for development of heart failure in women include hypertension and diabetes. Moreover, women have a higher risk for mortality than men if they suffer from left ventricular hypertrophy [42]. Evidence indicates that estrogen receptors in the heart modulate hypertrophy and subsequently, progression of heart failure. The composition of the vascular and cardiac extracellular matrix also contributes to the greater incidence of HFpEF in women, as well as Tako-Tsubo cardiomyopathy, a transient apical ballooning syndrome [43, 44]. Activational and organizational effects of hormones on cardiac remodeling that are adaptive to increases in cardiac output during pregnancy may be maladaptive in other circumstances, and the usual treatments directed towards increasing ejection fraction may not be appropriate for conditions when ejection fraction is preserved. Furthermore, it is unclear whether treatment guidelines for heart failure are applied to HFpEF, meaning women are not being started on renin–angiotensin inhibitors as often as those with depressed ejection fraction heart failure.
Ischemic Heart Disease and Microvascular Disease
Differences in response to myocardial injury between males and females are multifaceted and involve differences in contractile function, function of mitochondria, calcium metabolism, and cardiac growth [45]. Similar to hypertension, epidemiologic studies demonstrate that compared to men, premenopausal women have a reduced risk for ischemic heart disease. After menopause, this risk increases [46]. Ischemic heart disease in women is becoming recognized as having different etiology compared to obstructive disease characterized by occlusion of a large coronary artery at a single site [47]. Obstruction in women tends to be diffuse extending along the length of the artery [48, 49]. The risk factor profile for predicting development of ischemic disease may include factors other than those defined in the Framingham Risk Score (body mass index, total cholesterol, high density lipoprotein, systolic blood pressure, and smoking) [50] and include factors affecting ovarian function and pregnancy history [51–53]. Experimentally it has been shown that female sex favorably affects cardiac remodeling after reperfusion for ischemic injury. Sex steroid hormones are implicated in these differences. Interestingly though, cardiogenic shock is more common in women and women are less likely to present to revascularization-capable sites [54]. Moreover, women receive evidence-based therapy and coronary intervention less frequently than men demonstrating discrepancies in access or availability of care [46]. More research is needed to identify specific therapeutic strategies for women given identified underlying sex and gender differences.
Atherosclerosis and Other Inflammatory Associated Cardiovascular Condition
Propensity for inflammation may explain some of the sex differences in cardiovascular disease presentation. Atherosclerosis is an inflammatory disease provoked by biochemical sources such as lipid peroxidation and oxidative stress associated with metabolic syndrome [55], infection [56–58], or immunity-associated cellular activation [59, 60]. In general, inflammatory conditions in the general population demonstrate an increased propensity in women. For example, rheumatoid arthritis carries a 3.6 % prevalence in women and 1.7 % in men and Lupus has a 10:1 predominance in women. Most autoimmune disorders including Lupus are associated with increased risk of CV disease. However, some conditions, such as myocarditis, specifically fatal myocarditis, and myocardial fibrosis, have a male prevalence [61]. Additional research is needed to understand the mechanisms linking inflammatory conditions, immunity and cardiovascular disease, and preventive strategies in both men and women.
Thrombosis
Factors contributing to thrombosis are multifactorial and reflect not only genetic components but also the presence of infection, cancers, and mechanical injury [62, 63]. Biologically, these interactions were explained by Virchow as a triad of interaction of coagulability of the blood, blood flow, and anatomy of the vascular wall [63]. Coagulability of the blood reflects the type and amount of soluble proteins of the coagulation cascade and formed elements, that is, platelets, leukocytes, red blood cells, and cell-derived microvesicles [53]. Sex steroid hormones will affect production of proteins of the coagulation cascade and also the content of vasoactive and mitogenic factors in platelets by way of genomic actions in the liver and bone marrow megakaryocytes, respectively. Thus, it might be expected that hormonal shifts associated with pregnancy and menopause would affect risk for thrombosis. Indeed, incidence of thrombosis in the general population is age related and may be slightly greater in women of reproductive age compared to age-matched men and greater in older men than age-matched menopausal women [64]. Pregnancy may increase the risk for venous thrombosis but the factors contributing to risk may vary with each trimester reflecting hormonal changes on the blood components [65] and mechanical issues related to fetal and positional obstruction of venous flow.
Estrogenic menopausal treatments carry a black box warning for thrombotic risk. However, oral products may increase risk more than transdermal products due to the first pass effect on the liver and potential effects on the production of coagulation and inflammatory proteins in the liver apart from direct effects on platelets [66]. In ovariectomized experimental animals, estrogen treatments decrease platelet aggregation and secretion of mitogenic, vasoactive factors [67]. Similar observations need to be confirmed in postmenopausal women as data are emerging to support relationships between activated platelets and pro-thrombotic microvesicles in development of carotid intima medial thickening (associated with stroke risk) and development of white matter hyperintensities in the brain of postmenopausal women [68, 69].
Sex-Specific Conditions for Women
Pregnancy
Cardiovascular diseases associated with reproductive function are sex-specific. In men, erectile dysfunction is a sex-specific condition which is not life threatening but may be indicative of systemic atherosclerosis which could lead to myocardial infarction [70]. On the other hand, some cardiovascular-related diseases of pregnancy such as hypertensive disorders and spontaneous coronary artery dissection can predispose the affected woman to increased life-long risk for major adverse cardiovascular events (i.e., myocardial infarction, stroke, death) [71–75].
Given the major physiological adaptations of the cardiovascular systems needed to support fetal health, pregnancy has been described as a stress test on the cardiovascular system of women [76]. While most women “pass the test” without difficulty, others develop hypertensive disorders during the gestational period that are classified by time of gestation, magnitude of systolic blood pressure and the presence of protein in the urine such as gestational hypertension, preeclampsia, and eclampsia (Table 8.2) [53].
Table 8.2
Definitions of hypertensive pregnancy disorders
<20 GW | ≥20 GW | Delivery | ≥12 weeks post-partum | Diagnosis |
|---|---|---|---|---|
Normotensive | Hypertension without Proteinuria | Resolution of hypertension | Transient hypertension | |
Hypertension without Proteinuria | Persistent hypertension | Chronic (incident) hypertension | ||
Hypertension with Proteinuria | Resolution of proteinuria and hypertension | Preeclampsiaa |
It remains to be clarified as to whether pregnancy “unmasks” preexisting cardiovascular pathologies leading to hypertensive disorders that may increase a women’s life-long risk for cardiovascular disease or whether particular circumstances of the pregnancy such as ischemic-induced injury to the placenta, angiogenic growth factors, maternal endothelial dysfunction, and/or immunologically related cytokines cause the hypertensive disorder [71] (Fig. 8.1). In a retrospective cohort study, a modest association was found between a history of hypertensive pregnancy and increased frequency (odds ration [OR], 1.62; 95 % CI, 1.00–2.63), severity and duration of vasomotor symptoms in women suggesting perhaps an underlying physiological condition that may predispose a women to both conditions. However, after correction for potential confounders such as age, socioeconomic status, sedentary lifestyle, smoking, hormone use, body mass index, and hypertension, the significant association was lost indicating the need to further investigate the causal relationships between pregnancy-associated hypertension, hypertension development in women as they age and menopausal symptoms that have an autonomic regulatory component [77].
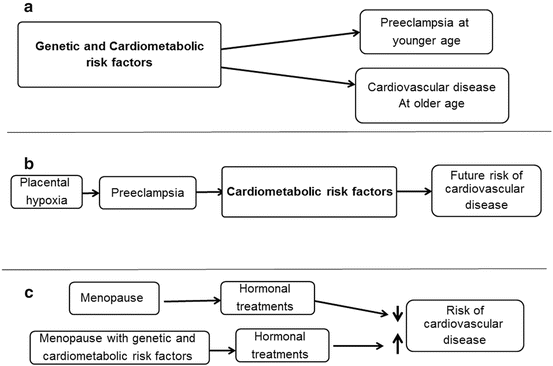

Fig. 8.1
Schematic of interaction of sex-specific conditions in women with cardiometabolic risk factors in establishing risk for development of cardiovascular disease. Cardiometabolic parameters include obesity, hypertension, diabetes, hyperlipidemia, inflammation, and infection. (a) The cardiometabolic factors predispose to development of preeclampsia, while in (b) preeclampsia initiates changes in the cardiometabolic profile that persists to increase life-long risk of cardiovascular disease. (c) Depicts two possibilities for disparate findings of effects of menopausal hormone treatments and risk for cardiovascular disease
Menopause
Menopause is the manifestation of decreased production of ovarian hormones and, thus, the loss of activational hormonal effects on the cardiovascular system. It would be reasonable, then, to propose that replacement or treatment of women with ovarian hormones would restore these activational effects and reduce risk of cardiovascular disease in menopausal women. Indeed, estrogenic treatments reduce vasomotor symptoms of menopause and multiple observational studies demonstrate reduced incidence and all-cause cardiovascular disease mortality in menopausal women using such treatments for symptom relief [78–85]. However, observational studies are criticized for demonstrating healthy user bias.
The Women’s Health Initiative (WHI) was designed as a prospective randomized, placebo controlled trial to evaluate risk of cardiovascular events in postmenopausal women. Treatments were conjugated equine estrogen alone or in combination with medroxyprogesterone acetate. The results of this trial were surprising in that the number of cardiovascular events was greater rather than fewer in the treated groups compared to placebo [86]. Important to note is that women in the WHI were greater than a decade past menopause (mean age about 63 years) and not representative of women using hormone treatments for menopausal symptoms as was the case in many of the observational studies [83, 85].
Results from basic science studies support the hypothesis that timing of initiation of hormonal treatments affects the outcomes. In studies of nonhuman primates, hormone treatments started at the time of ovariectomy reduced coronary artery atherosclerosis, whereas initiation of treatment 2 years later did not [87]. Thus, timing of initiation of hormonal treatments may contribute to their biological effects [88]. In terms of the classification of hormonal effects, there may be a limited period in which activational hormonal effects can be reversed. This period of opportunity may vary by cell type and the temporal manifestation of the physiological effects may exceed the duration of the treatment itself.
There are several studies which support these hypotheses. The Danish Osteoporosis Prevention Study randomized recently postmenopausal women to hormone treatment or placebo and found that after 10 years women receiving hormonal treatments had a significantly reduced risk (hazard ratio 0.48, 95 % confidence interval 0.26–0.87; P = 0.015) of cardiovascular events such as myocardial infarction and heart failure with no increased risk of venous thromboembolism, cancer, or stroke [89]. However, in a subgroup analysis of women closer to menopause (50–59 years of age), cardiovascular events were fewer in the treated groups compared to placebo. In addition, in long-term follow-up of women in the WHI, decreases in coronary heart disease and stroke in women randomized to conjugated equine estrogen were not observed until about 7 years after cessation of treatment [90, 91].
Meta-analysis of epidemiological studies of women who had early menopause by oophorectomy and used hormonal treatments suggest that reversible activational effects of hormonal treatments might be tissue specific. Treatments initiated and used until the age of natural menopause seem to be protective against stroke but use of the treatments for years beyond the time of natural menopause increased the risk of stroke [92].
Hormonal treatments may interact with other metabolic parameters that contribute overall cellular viability and thus influence disease risk (Fig. 8.1c) [93]. For example, in the WHI, women meeting criteria for metabolic syndrome had an increased risk for adverse cardiovascular events with estrogenic treatments [94]. Furthermore, as estrogens affect gene methylation, genetic variants that may associate with disease parameters in hormone replete or deplete conditions may not do so in the reverse situation.
Conclusion
Cardiovascular disease mortality for women exceeds that of men for many reasons including, but not limited to, biological differences due to sex chromosomes and both activational and organization effects of the sex steroid hormones. Underlying physiological regulatory mechanisms of the cardiovascular system differ in women compared to men resulting in sex differences in prevalence and presentation of cardiovascular conditions including those associated with autonomic regulation, development of hypertension, and conditions associated with vascular and cardiac remodeling. Hormonal mechanisms drive cardiovascular changes in two conditions unique to women, pregnancy and menopause. However, much remains to be learned regarding potential causal relationships among cardiovascular challenges associated with pregnancy and menopause and progression of cardiovascular disease in women across their life span in order to better define risk stratification, monitoring and treatments to reduce the disparity in cardiovascular mortality between women and men.
References
1.
2.
3.
4.
Biino G, Parati G, Concas MP et al (2013) Environmental and genetic contribution to hypertension prevalence: data from an epidemiological survey on Sardinian genetic isolates. PLoS One 8(3):e59612PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree