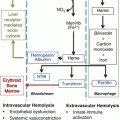
Fig. 13.1
Risk of postoperative infection after transfusion of non-WBC-reduced allogeneic RBCs or whole blood, as compared with transfusion of WBC-reduced allogeneic RBCs or whole blood. Randomized controlled trials (RCTs) of ABT and postoperative infection, depicted on a logarithmic scale and in the order of their publication or presentation, and administering either buffy-coat-rich or buffy-coat-reduced allogeneic RBCs or whole blood to the non-WBC-reduced arm, and either pre-storage or post-storage-filtered allogeneic RBCs or whole blood to the WBC-reduced arm [31–42]. The figure shows the odds ratio (OR) of postoperative infection in recipients of non-WBC-reduced versus WBC-reduced allogeneic RBCs or whole blood, as calculated from an intention-to-treat analysis of each study. Although the heterogeneity among the 12 available RCTs precludes any integration of their findings by the methods of meta-analysis, the figure shows a summary OR calculated by the random-effects method of DerSimonian–Laird solely for the purpose of illustration. A deleterious ABT effect (and thus a benefit from WBC reduction) across the 12 RCTs would be demonstrated by an OR > 1, provided that the effect were statistically significant (p < 0.05; i.e., provided that the associated 95 % confidence interval [CI] of the summary OR did not include the null value of 1). No such effect is detected here because the 95 % CI of the summary odds ratio includes the null value of 1. More specifically, with the caveat that this exercise reflects no real biologic effect owing to the medical heterogeneity of the studies, when all 6290 patients randomized in the 12 available RCTs are included in intention-to-treat analyses of the 12 studies, no TRIM effect is detected (summary OR = 1.24; 95 % CI, 0.98–1.56; see figure). Similarly, when only the 4460 actually transfused patients are retained in “as-treated” analyses of the 12 studies, again no TRIM effect is detected (summary OR = 1.31; 95 % CI, 0.98–1.75; data not shown) [1, 30]
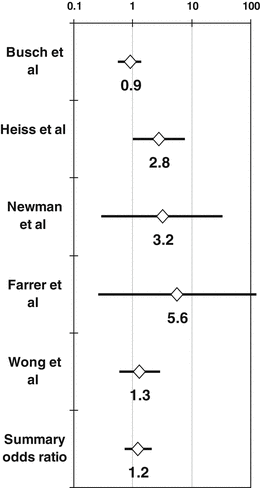
Fig. 13.2
Risk of postoperative infection after transfusion of allogeneic RBCs, as compared with transfusion of autologous RBCs or whole blood. Randomized controlled trials (RCTs) of ABT and postoperative infection, depicted on a logarithmic scale and administering either buffy-coat-rich or buffy-coat-reduced allogeneic RBCs or whole blood to the allogeneic arm, and either autologous RBCs or whole blood obtained by preoperative blood donation [46, 47] or blood procured by ANH, IBR, or PBR to the autologous arm [48–50]. The figure shows the odds ratio (OR) of postoperative infection in recipients of allogeneic versus autologous RBCs or whole blood, as calculated from an intention-to-treat analysis of each study; as well as a summary OR across the 5 RCTs, as calculated from a meta-analysis [51]. There is no deleterious ABT effect (and thus no benefit from autologous transfusion), because the 95 % CI of the summary odds ratio includes the null value of 1 [51]
Patients randomized to receive autologous or WBC-reduced allogeneic RBCs received units that were either replete with or devoid of WBC-derived soluble mediators. During storage of a non-WBC-reduced RBC unit, WBCs deteriorate over 2 weeks, progressively releasing soluble mediators. RBC units that are WBC-reduced by filtration following storage are still replete with WBC-derived mediators because these mediators (in addition to apoptotic or necrotic WBCs) are not removed by WBC reduction filters. RBCs that are WBC-reduced by filtration prior to storage are free of WBC-derived mediators, because their WBCs are removed before they can release mediators into the supernatant fluid [30]. WBCs in stored autologous blood, obtained by preoperative donation, also deteriorate during storage and release mediators. Fresh autologous blood, obtained by acute normovolemic hemodilution (ANH) , intraoperative blood recovery (IBR) , or postoperative blood recovery (PBR) , and transfused within hours of collection and processing , is free of WBC-derived soluble mediators [51].
WBC reduction, performed either before or after storage, can prevent TRIM effects mediated by allogeneic mononuclear cells (AMCs) , but it cannot prevent TRIM effects mediated by soluble molecules circulating in allogeneic plasma. Only pre-storage, as opposed to post-storage, WBC reduction can prevent TRIM effects mediated by WBC-derived, soluble mediators. Autologous transfusion can prevent TRIM effects mediated by AMCs as well as by soluble molecules circulating in allogeneic plasma. Only fresh, as opposed to stored, autologous blood can prevent TRIM effects mediated by WBC-derived, soluble mediators [30, 51].
TRIM Effects Mediated by Allogeneic Mononuclear Cells
The only established TRIM effect (i.e., the beneficial effect of pretransplant ABT on renal allograft survival) appears to require viable WBCs. Patients awaiting renal transplantation derived less immunologic benefit from pretransplant RBC transfusions that are WBC-reduced, washed, or frozen-thawed. Mincheff et al. [52] implicated the dendritic antigen-presenting cells (APCs) of the allogeneic donor in the induction of a state of anergy in the recipient, proposing that during refrigeration APCs lose their ability to deliver co-stimulation. These investigators hypothesized that, following ABT, the recipient’s T cells are stimulated by allogeneic donor APCs in the absence of co-stimulation, and this interaction induces a state of anergy in the recipient’s T cells.
Animal data suggest that TRIM effects are most likely mediated by transfused allogeneic mononuclear cells [53]. Kao [54] induced immune suppression in mice receiving donor WBCs free of plasma and platelets. A recent theory [55] proposes that donor dendritic cells expressing both alloantigen and the OX-2 (CD200) co-stimulatory molecule are required for the production of the TRIM effect .
Clark et al. [55] demonstrated ABT -induced tumor growth in a murine model that employed BALB/c mice as allogeneic donors and C57B1/6 mice as blood recipients. The recipient mice received a tail vein injection of syngeneic FSL10 fibrosarcoma cells, followed by transfusion of 50–200 μL of allogeneic blood. Pulmonary tumor nodules were counted 3 weeks after the FSL10 cell infusion. There was a dose-response relationship between the volume of transfused allogeneic blood and the number of pulmonary tumor nodules, along with proliferation of TGF-β-positive suppressor T-cells in the spleen.
Donor myeloid dendritic cells that expressed both CD11c and CD200 on their surface appeared to mediate the tumor-growth-promoting effect of ABT , as the effect could be blocked by monoclonal antibodies to either CD11c or CD200. However, the effect could not be blocked by antibodies to CD200R, an observation that implicated a subset of donor myeloid dendritic cells expressing both CD11c and CD200 in the pathogenesis of TRIM. The interaction between the donor CD200 and presumably the cognate receptor on the recipient’s T cells induced proliferation of γδ-suppressor T cells that released cytokines, especially TGF-β. Physiological concentrations of TGF-β stimulated proliferation of FSL10 fibrosarcoma cells in vitro. As TGF-β can also suppress host defenses against infectious agents, it could be the basis of the TRIM effect with regard to both postoperative infections and tumor growth, at least with regards to sarcomas.
Bordin and Blajchman [56] reviewed the findings of animal models of ABT and cancer recurrence and reported that 17 published models had found stimulation of tumor growth by ABT, as compared with three models that had reported inhibition of tumor growth and 4 models that had found no effect. Data from both inbred and outbred animal models have indicated that ABT accelerates tumor growth and enhances formation of metastatic nodules [57–60]. Allogeneically transfused mice inoculated intramuscularly with either syngeneic malignant melanoma (B16) or mastocytoma (P815) cells developed larger tumors than did syngeneically transfused mice [58]. Similar results were obtained when syngeneic B16 tumor cells were infused intravenously and the numbers of pulmonary nodules enumerated [58, 59]. Experiments performed to investigate the effect of the tumor-cell dose showed that the ABT effect was only evident when small numbers (1.25–2.5 × 105) of tumor cells were inoculated into the host animal. The effect was not evident when larger numbers of tumor cells were inoculated, suggesting that the tumor burden had a strong bearing on whether the ABT effect became manifest .
Studies in both inbred (mice) and outbred (rabbits) animals have shown that ABT has a tumor-growth-promoting effect when administered prior to the infusion of syngeneic tumor cells [57, 60]. In the murine model, male C57Bl/6J mice (MHC type H-2b) were blood recipients; Balb/c mice (MHC type H-2d) were allogeneic donors; and the tumor cells were syngeneic (H-2b) methylcholanthrene-induced fibrosarcoma cells [60]. To better replicate the situation seen clinically, the enhancement of tumor growth by ABT was investigated in animals (mice and rabbits) that received such syngeneic and allogeneic transfusions subsequent to the inoculation of the tumor cells, and the data indicated that ABT-enhanced tumor growth also occurs in animals with established tumors [57]. Is was also shown that animals with either non-established or established tumors receiving non-WBC-reduced ABT developed significantly larger numbers of pulmonary nodules than did animals given WBC-reduced ABT [57, 60].
Finally, the tumor-growth-promoting effect of ABT can be adoptively transferred to naive animals by spleen cells harvested from allogeneically transfused animals [60]. In these experiments, the number of pulmonary nodules observed in animals that had received spleen cells from allogeneically transfused animals was significantly higher than that observed in animals that had received spleen cells from animals transfused with syngeneic blood. Importantly, the ABT effect could not be adoptively transferred to naive animals that received spleen cells derived from animals transfused with pre-storage-WBC-reduced allogeneic blood. Despite convincing results from animal models regarding tumor-promoting effects of non-WBC-reduced ABT [53–60], the findings of RCTs investigating the association between non-WBC-reduced ABT and cancer recurrence in humans [32, 46, 47] have been negative.
The only RCT to study the effect of AMCs has been the Viral Activation Transfusion Study, which studied transfusion-induced activation of endogenous CMV or HIV infection [45]. All RBCs transfused in this study had been stored for less than 2 weeks and could thus be presumed to contain immunologically competent AMCs. There was no difference between the arms of the RCT in HIV-RNA level, the number of CD4-positive T cells, or any other end-point studied. Median survival was 13.0 months in recipients of pre-storage filtered, WBC-reduced allogeneic RBCs, as compared with 20.5 months in recipients of buffy-coat-rich, non-WBC-reduced allogeneic RBCs. This difference was not siginificant in the intention-to-treat analysis (p = 0.12) but, after adjustment for various prognostic factors, transfusion of non-WBC-reduced RBCs was associated with a better outcome.
TRIM Effects Mediated by White-Cell-Derived Soluble Mediators
Soluble immune response modifiers accumulating during storage of blood components include elastase, histamine, soluble HLA, soluble Fas ligand, transforming growth factor (TGF)-β1 and proinflammatory cytokines IL-1β, IL-6, and IL-8. In vitro, soluble WBC-derived factors from stored RBCs induce immediate up-regulation of expression of inflammatory genes in third-party WBCs. Apoptosis of WBCs begins immediately after the collection of donor blood. Gradual apoptosis and necrosis begins with granulocytes and continues with monocytes, while lymphocytes can remain viable for >25 days at 2–6 °C. Apoptotic cells engage the phosphatidylserine/annexin-V receptor on macrophages, inducing release of prostaglandin E-2 and TGF-β—factors that suppress macrophages and natural-killer cells and impair antigen-presenting capacity.
However, the 12 RCTs that compared the risk of postoperative infection between patients randomized to receive non-WBC-reduced versus WBC-reduced ABT (in the event that they needed perioperative transfusion) have not supported the theory that attributes TRIM to WBC-derived soluble mediators. These RCTs are medically heterogeneous, having been conducted at various settings, having transfused various blood products, and having diagnosed infection based on varying criteria. Thus, not all 12 RCTs targeted a TRIM effect that was biologically similar in all cases, making it inappropriate to combine the results of all 12 RCTs in a meta-analysis [1, 30]. However, if we were to integrate these findings despite the extreme heterogeneity of the studies, we would find no association between non-WBC-reduced ABT and an increased risk of infection across all the available RCTs, whether we relied on “intention-to-treat” analyses (that retain all randomized subjects, whether transfused or not) or on “as-treated” analyses (that remove the untransfused subjects—Fig. 13.1).
What has medical relevance, however, is the integration of medically homogeneous studies. Integration of such subsets of homogeneous studies produced results antithetical from those expected from theory. Across 9 RCTs transfusing allogeneic RBCs filtered before storage to the WBC-reduced arm, and enrolling approximately 5000 subjects, no TRIM effect was detected. If WBC-derived, soluble mediators did cause TRIM, pre-storage filtration should abrogate any increased infection risk associated with non-WBC-reduced ABT . Thus, a deleterious TRIM effect would be expected in this analysis, but no such effect was found (summary odds ratio [OR] = 1.06; 95 % confidence interval [CI], 0.91–1.24; p > 0.05) [30].
In contrast, across the 4 RCTs transfusing allogeneic RBCs or whole blood filtered after storage to the non-WBC-reduced arm, there was a 2.25-fold increase in the risk of infection in association with non-WBC-reduced ABT (summary OR = 2.25; 95 % CI, 1.12–4.25; p < 0.05) [30]. Thus, the TRIM effect appeared to be prevented by post-storage filtration, contradicting the theory that attributes TRIM to WBC-derived, soluble mediators. Such mediators would have been present equally in both the non-WBC-reduced and WBC-reduced RBCs, because they would not have been removed by post-storage filtration.
TRIM Effects Mediated by Soluble Molecules Circulating in Allogeneic Plasma
Only 1 RCT has been specifically designed to study the effects of soluble HLA molecules circulating in allogeneic plasma as mediators of TRIM. Wallis et al. [38] randomized patients undergoing open-heart surgery to receive plasma-reduced, buffy-coat-reduced, or WBC-reduced RBCs. The highest risk of infection was observed in the plasma-reduced arm, although the difference between the three arms was not significant. This finding suggested that plasma removal does not prevent TRIM; or, by extension, that allogeneic plasma does not mediate TRIM. Similarly, integration of the 5 RCTs [46–50] that compared recipients of allogeneic versus autologous blood demonstrated no increased risk of infection in association with ABT [51], whether the analysis included all studies (Fig. 13.2) or was limited to RCTs transfusing blood obtained through ANH, IBR, or PBR to the autologous arm of the studies [48–50].
Association of Non-white-cell-reduced Allogeneic Blood Transfusion with Postoperative Mortality
The association of non-WBC-reduced ABT with short-term mortality from all causes started out as a data-derived hypothesis to account for an unexpected transfusion effect. The RCT of van de Watering et al. [34] had been designed to investigate differences in postoperative infection between recipients of non-WBC-reduced versus WBC-reduced allogeneic RBCs. However, it detected, instead, differences in 60-day mortality between the arms (Fig. 13.3). The authors suggested that non-WBC-reduced ABT may predispose to MOF, which, in turn, may predispose to mortality.
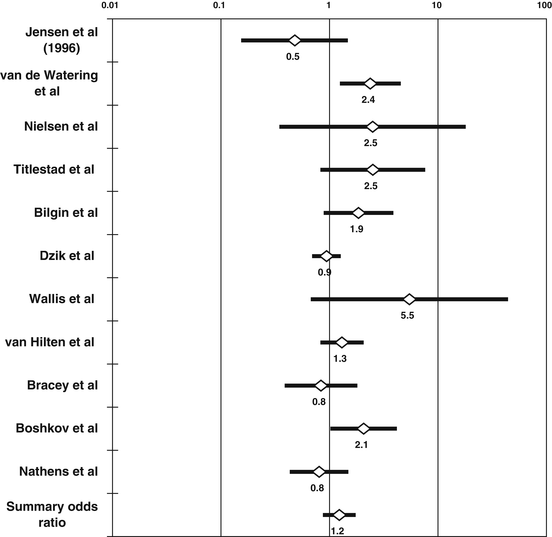

Fig. 13.3
Postoperative mortality after transfusion of non-WBC-reduced allogeneic RBCs, as compared with transfusion of WBC-reduced allogeneic RBCs. Randomized controlled trials (RCTs) investigating the association of non-WBC-reduced allogeneic blood transfusion (ABT ) with short-term (up to 3-months post-transfusion) mortality from all causes, depicted on a logarithmic scale and in the order of their publication or presentation, and administering either buffy-coat-rich or buffy-coat-reduced allogeneic RBCs to the non-WBC-reduced arm, and either pre-storage or post-storage-filtered allogeneic RBCs to the WBC-reduced arm [33, 34, 36–44]. For each RCT, the figure shows the odds ratio (OR) of short-term mortality in recipients of non-WBC-reduced versus WBC-reduced allogeneic RBCs, as calculated from an intention-to-treat analysis of each study. Although the heterogeneity among the 11 available RCTs precludes their integration by the methods of meta-analysis, the figure shows a summary OR calculated by the random-effects method of DerSimonian–Laird solely for the purpose of illustration. There is no deleterious ABT effect (and thus no benefit from WBC reduction), because the 95 % CI of the summary odds ratio includes the null value of 1 [1]
If 11 medically heterogeneous RCTs reported before 2005 and comparing recipients of non-WBC-reduced versus WBC-reduced ABT and reporting on short-term mortality [33, 34, 36–44] were to be combined, there would be no increase in mortality in association with non-WBC-reduced ABT (Fig. 13.3) [1]. These studies transfused to the non-WBC-reduced arm either buffy-coat-rich or buffy-coat-reduced allogeneic RBCs; as already discussed, the former should have more of an effect than the latter. However, this theoretical prediction is the opposite of what the analysis actually showed. Across 6 RCTs transfusing buffy-coat-reduced RBCs to the non-WBC-reduced arm, and pre-storage filtered RBCs to the WBC-reduced arm, there was a 60 % increase in mortality in association with non-WBC-reduced ABT (summary OR = 1.60; 95 % CI, 1.14–2.24; p < 0.05) [1]. In this analysis, pre-storage filtration appeared to abrogate an increased mortality risk, but the benefit from pre-storage filtration was not seen where more of an ABT effect would have been expected. Across the RCTs that transfused buffy-coat-rich RBCs to the non-WBC-reduced arm, no ABT effect was detected, although some 4500 subjects had been enrolled in these studies.
Perhaps, the benefit observed in the analysis of studies transfusing buffy-coat-reduced versus pre-storage filtered RBCs was due to overrepresentation in that analysis of the cardiac surgery studies: three of the six RCTs included in that analysis (i.e., the studies by van de Watering et al. [34], Bilgin et al. [37], and Wallis et al. [38]) had been conducted in open-heart surgery. Across all 5 RCTs conducted in cardiac surgery [34, 37, 38, 40, 41] (Fig. 13.3), there was a 72 % increase in mortality in association with non-WBC-reduced ABT (summary OR = 1.72; 95 % CI, 1.05–2.81; p < 0.05). In contrast, across the remaining 6 RCTs conducted in other surgical settings (Fig. 13.3), there was no ABT effect (summary OR = 0.99; 95 % CI, 0.73–1.33; p > 0.05) [1].
Thus, the ABT -related mortality risk, which is not seen in any other setting, may relate to another effect present in patients undergoing cardiac surgery . During open-heart surgery, blood is exposed to the extracorporeal circuit, as well as to hypothermia and to ischemic and reperfusion injury. These insults are potent inducers of a stress response, triggering a systemic inflammatory response syndrome (SIRS), which is immediately counteracted by a compensatory anti-inflammatory response syndrome (CARS) [61]. SIRS manifests with leukocytosis, capillary leakage, and organ dysfunction; overwhelming SIRS causes a dormant state of metabolism referred to as multiple-organ-dysfunction syndrome (MODS) . CARS has an immune-paralyzing effect characterized by anti-inflammatory cytokines, such as TGF-β1, IL-4, and IL-10. Through the intervention of CARS, the post-perfusion SIRS of cardiac surgery generally resolves. However, any intervention by biologic response modifiers during an already-existing inflammatory cascade can push the SIRS/CARS equilibrium toward SIRS, thereby leading to MODS, MOF, and death. WBC-containing ABT administered during cardiac surgery may provide this “second hit,” exacerbating the SIRS and potentially causing the patient’s death [61]. However, this explanation is speculative as, hitherto, no cardiac-surgery RCT has reported an association between non-WBC-reduced (versus WBC-reduced) ABT and MOF. In the completed cardiac-surgery RCTs, non-WBC-reduced ABT was not associated with any particular cause of death, yet the aggregate mortality was higher in the non-WBC-reduced arm than in the WBC-reduced arm [1].
Bilgin et al. [62] investigated the pro- and anti-inflammatory cytokine profiles in patients participating in their cardiac-surgery RCT that compared recipients of buffy-coat-reduced versus WBC-reduced allogeneic RBCs [37]. Patients who developed postoperative infection had higher IL-6, and patients who developed MODS had higher IL-12 concentrations, in the subgroup of subjects who received more than three non-WBC-reduced RBC units. These findings supported the authors’ thesis that non-WBC-reduced ABT amplifies an inflammatory response which is a “second hit” superimposed upon the on-going SIRS induced by cardiac surgery . Such a “second-hit” inflammatory response may subsequently lead to a more profound CARS which amounts to transfusion-induced immunosuppression predisposing to enhanced susceptibility to postoperative infection .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree