Patients specific
Iatrogenic
Age >70
Excessive transfusions
Renal failure
Infusion rate >225 mL/h
Congestive heart failure
Increased volume of transfusion
Hemorrhagic shock
Positive fluid balance
Severe liver disease
Prolonged surgical time
Cardiac surgery
Vascular surgery
Liver surgery
In addition to intrinsic patient risk, multiple iatrogenic factors contribute to the risk of developing TACO. These include a greater number of transfusions given, increased rate of transfusion, and volume of the transfusate [7, 12]. In TACO cases, the number of units transfused varies from 1.8 to 4 units but can be as few as one [2, 10, 11, 13]. In a prospective cohort study, a transfusion rate of 225 mL/h compared to 168 mL/h was associated with increased risk of TACO (Odds Ratio (OR) 1.88, 95 % confidence interval (95 % CI) of 1.06–3.33) [10], while another found that a mean positive fluid balance in patients transfused who later developed TACO was +4.7 L compared with 2.75 L in controls [OR 1.18 (95 % CI 1.08–1.28)]. The positive fluid balance per hour was +0.38 L vs. +0.26 L, respectively [OR 3.6 (95 % CI 1.52–8.5)] [12]. The amount of volume per transfusion can vary based on blood type, amount donated, and center transfusing but the approximate volume of red cells or FFP is 200–400 mL per transfusion [14, 15].
Clinical Presentation
The clinical features of TACO consist of new onset respiratory distress, hypoxemia, orthopnea, and/or cough during or after blood transfusion. Physical exam findings may include jugular venous distention, rales, and peripheral edema. Patients may exhibit abnormal vital signs such as elevated heart rate, systolic and diastolic blood pressure, respiratory rate, and lower oxygen saturation, compared to those before transfusion [7].
Pathophysiology
The driving pathophysiology in TACO is an increase in intravascular fluid, leading to elevated hydrostatic pressure in the pulmonary vasculature, and promoting fluid accumulation in the lung. Fluid dynamics and handling drive the development of pulmonary edema, contrasting with the priming, inflammation, and capillary leak occurring in Transfusion Related Acute Lung Injury (TRALI) [16].
In the lung, the main forces contributing to fluid accumulation are hydrostatic pressures, oncotic pressures, and integrity of the capillary interface, otherwise known as Starling Forces [17]. As hydrostatic pressures in the lung increase relative to oncotic forces, more fluid is forced into the interstitium. Normal or marginally elevated hydrostatic pressures in the setting of low oncotic pressure may cause the same effect such has in hypoalbuminemia. It is unclear whether the hypervolemic effect of blood products may be amplified by their increased extrapulmonary oncotic pressure compared to that of isotonic fluid [18].
In critically ill patients, an overlap syndrome may exist between TRALI and TACO [19]. In these critically ill patients, disruption of the capillary interface is thought to magnify the effect of elevated hydrostatic pressures (Fig. 12.1). A study evaluating the differences between pulmonary edema secondary to cardiogenic and noncardiogenic etiologies noted a more positive relationship between elevated PAOP and amount of “lung water” in Acute Respiratory Distress Syndrome (ARDS) compared with cardiogenic etiologies [20]. This relationship was again noted in The Fluid and Catheter Treatment Trial (FACTT) which investigated two different fluid strategies in ARDS patients [21].
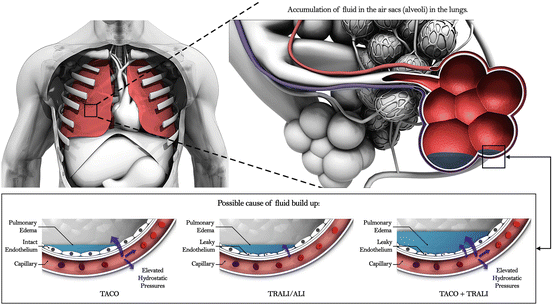

Fig. 12.1
Transfusion associated circulatory overload (TACO) represents movement of fluid into alveoli secondary to hydrostatic pressure. Transfusion related acute lung injury/acute lung injury (TRALI/ALI) occurs when the integrity of the endothelial interface is compromised. TACO and TRALI/ALI may occur concurrently with pulmonary edema occurring from both leaky endothelium and elevated hydrostatic pressures. Courtesy of Peter L. Gibfried
Diagnostic Evaluation
While no single test can defines the diagnosis of TACO, several are highly suggestive (Fig. 12.2). As the mechanism driving TACO is elevated cardiac filling pressures secondary to excess volume, diagnostic techniques can be used for its identification. The pulmonary artery catheter is one of the most direct modalities to measure elevated cardiac filling pressures. In the setting of transfusion related pulmonary edema, the finding of a pulmonary artery occlusion pressure (PAOP) greater than 18 mmHg can suggest TACO versus TRALI which would be below 18 mmHg [22]. While once a mainstay guiding ICU therapy, for a variety of reasons, the pulmonary artery catheter has fallen out of favor in recent practice [23].
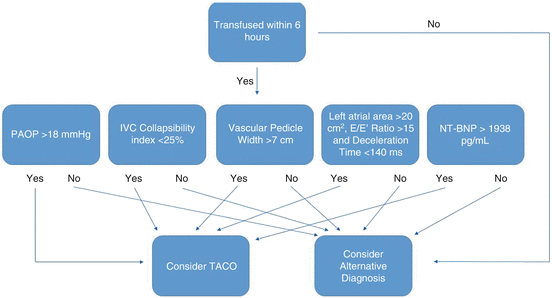

Fig. 12.2
Diagnostic algorithm for TACO
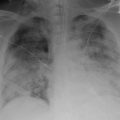
One of the simplest and most informative studies performed for the investigation of TACO is the chest roentgenogram (X-ray). Pulmonary edema can be visualized in the lung fields and is characterized by perihilar opacities and bibasilar effusions. Additionally, elevated cardiac pressures may be inferred from findings of cephalization, pleural effusions, and the increased vascular pedicle width on chest X-ray. The measurement of vascular pedicle width has been studied and correlated to elevated PA catheter wedge pressures [24]. The technique is performed by making a perpendicular line from left subclavian artery exit of the aortic arch and measuring across to the point at which the superior vena cava crosses the right main stem bronchus [25]. A vascular pedicle width >7 cm has a 56 % sensitivity and 74 % specificity for a PAOP >18 mmHg. In situations in which echo is unavailable and PA catheter is not being considered, this may be a simple and useful strategy to suggest fluid overload in a patient receiving blood transfusion.
Bedside ultrasound and echocardiography are increasingly common in ICU practice. Evaluation of the thorax can be rapidly performed in a patient with acute respiratory distress using ultrasound. Multiple anterior diffuse B-lines may be suggestive of pulmonary edema with sensitivity of 97 % and specificity of 95 % [26]. Identification of pleural effusions can be made by sitting the patient upright and directing the ultrasound superior to the costovertebral angle. Simple anechoic fluid may be suggestive of pleural effusion and fluid overload. Although these findings are seen in pulmonary edema of any etiology, left heart failure or elevated central venous pressures are prerequisite for the diagnosis TACO.
In recent years, interest in the noninvasive evaluation of intravascular fluid status using ultrasound and echocardiography has grown. Many iterations of the ultrasound exam have been devised for this purpose, with respirophasic inferior vena cava (IVC) variation being the most widely studied. While this technique has not been evaluated in its diagnostic accuracy for TACO, it may be extrapolated that the determination of intravascular fluid balance in a recently transfused patient with pulmonary edema can be helpful in determining the potential etiology of respiratory distress. Respirophasic IVC variation is measured by directing a phased array ultrasound probe just inferior to the xiphoid process 1–2 cm to the right with marker dot position toward the operator. The probe can then be angled perpendicularly to the patient and then adjusted to angle cephalad. The operator can then sweep the probe until the IVC is visualized longitudinally. The ultrasound can then be switched to M-mode and evaluation of the IVC diameter during respirophasic variation can be made.
This modality has been investigated in both intubated [27] and non-intubated [28] patients for the purpose of determining fluid responsiveness. The IVC collapsibility index (IVC-CI) defined as the percent change between IVC diameter at end expiration and end inspiration has been inversely correlated. 90 % of patients with IVC-CI of <25 % will have a CVP of >7 mmHg while 85 % of patients with IVC-CI >75 % will have a CVP of 0–6 mmHg [29].
In addition to IVC parameters, echocardiographic parameters have also been associated with elevated PAOP. These include: mitral E/E′ ratio, left atrial area, and deceleration time. The mitral E/E′ ratio is the ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity which is a marker of end diastolic filling pressures [30]. Deceleration time is the time from peak flow to stasis across the mitral valve. These parameters are associated with PAOP of >15 mmHg for a left atrial area of ≥20 cm2, a mitral E/E′ ratio of ≥15, and a deceleration time <140 ms. The sensitivity and specificity for each was 66 and 89 % for mitral E/E′ ratio, 55 and 96 % for left atrial area and 51 and 93 % for deceleration time, respectively. If all three tests were used, the sensitivity and specificity was 92 and 85 %, respectively. Lastly, if all were positive, the odds ratio for PAOP >15 mmHg was 48 (CI 10–289, p < 0.001) [31].
Laboratory tests may also suggest the diagnosis. One of the most studied biomarkers in the determination of TACO is N-terminal Brain Natriuretic Peptide (NT-BNP) . NT-BNP was initially isolated from porcine neural tissue. It is released in humans in response to myocardial stretch and counteracts the renin-angiotensin system to reduce excess salt and water retention as well as inhibit vasoconstriction, promote vascular relaxation, and reduce sympathetic outflow [32].
In non-ICU populations, the marker had sensitivity and specificity of 87.5 and 95.8 %, respectively, for a level of >1923 pg/mL [33]. In patients with TACO, pre- and post-N-terminal BNP (NT-BNP) levels are elevated compared to controls who received transfusion and did not have TRALI or TACO [33]. A prospective cohort study evaluating the utility of BNP and NT-BNP in differentiating between TRALI and TACO in ICU patients found that levels differed significantly between entities. The area under the curve was 0.63 (95 % confidence interval [CI] 0.51–0.74) and 0.70 (95 % CI 0.59–0.80) for BNP and NT-BNP, respectively [34]. As such, the use of this biomarker may be limited in differentiating TRALI from TACO in an ICU population [35].
Several additional biomarkers have been investigated including pulmonary fluid protein concentration, radiolabeled albumin and interleukins (IL) 6, 8 and 10 with variable success. Previous studies have revealed that TRALI patients will have elevated IL-6 and 8 levels prior to the event relative to controls. A recent nested case–control study evaluating the use of IL-6, 8, 10, tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony stimulating factor (GM-CSF) were used to distinguish TACO versus TRALI [36]. While in multivariate analysis a good correlation existed between these levels might distinguish TRALI from TACO, but in univariate analysis specificity was only 59 % compared with 90 % in multivariate analysis. At this time, insufficient data exist to recommend routine clinical use of these assays [37–39].
Prevention
Recognition of populations at higher risk as well as modifiable risk can guide not only early identification of TACO, but also in its prevention. As always, the decision to transfuse should be given proper deliberation. An ever-growing body of data indicates the need for judicious use of blood transfusion at lower transfusion thresholds [40–42]. The best way to prevent any transfusion reaction is to not transfuse at all. Like all clinical interventions, the decision to transfuse should occur only after assessing that the benefits of transfusion outweigh the risks.
Older patients, those with heart failure, renal failure, and hemorrhagic shock should be carefully monitored when receiving blood products. Clinical suspicion for TACO should remain high. Careful monitoring of intake and outtake recording is important. Attention should also be paid to the patient’s fluid balance, both for the day of transfusion and cumulatively. Physical examination should be recorded both before and after transfusion as initial signs might be subtle. These include increased respiratory distress, elevated blood pressure, tachypnea, hypoxemia, elevated JVD, third heart sounds, crackles, decreased breath sounds, or edema. Transfusion of single units should be considered for high risk patients. While The American Association of Blood Banks (AABB) Technical Manual 17th edition recommends a transfusion rate of 240 mL/h, a rate of 168 mL/h (as discussed above) has been associated with reduced risk of TACO [10] and should be considered for high risk patients.
While definitive data for prophylactic diuretic use are lacking, these may be of benefit. A recent Cochrane Review, including four randomized controlled studies, found a decrease in PAOP and improvement in fraction of inspired oxygen with diuretics prior to transfusion. However, no differences were noted in clinically meaningful endpoints defined as acute respiratory distress, tachycardia, increased blood pressure or acute/worsening pulmonary edema on chest X-ray [43].
Treatment
Acute Support
Goals of acute management are consistent with the general support of the patient with volume overload of any type. Airway, breathing, and circulation should be addressed first. The transfusion should be stopped immediately. Supplemental oxygen, noninvasive or invasive ventilation should be considered. While high flow oxygen is an option [44], given the pathophysiology of TACO, noninvasive positive pressure ventilation may be more appropriate [45]. In patients with systolic dysfunction, preload and afterload reduction should be initiated. This can be performed using nitroglycerine, angiotensin converting enzyme inhibitors. Morphine might also be used to reduce dyspnea and provide further preload reductions, similar to its use in patients with acute heart failure [46] (Table 12.2).
Table 12.2
Treatment modalities
Supportive care
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|