TRALI
– Acute onset within 6 h after a blood transfusion
– PaO2/FiO2 <300 mmHg
– Bilateral infiltrative changes on the chest X-ray
– No sign of hydrostatic pulmonary edema (PAOP <18 mmHg or CVP <15 mmHg)
– No other risk factor for ALI present
Possible TRALI
– Other risk factor for ALI present
Delayed TRALI
– Onset of TRALI between 6 and 72 h after a blood transfusion
In this chapter, we summarize available knowledge on the pathogenesis and potential mechanisms of the relation between transfusion and ARDS , highlighting both the role of the recipient as well as of the blood product. We describe both TRALI as well as the association between transfusion and ARDS which does not fulfill TRALI criteria, with the aim to integrate pathophysiology in an explanatory model.
Transfusion and ARDS in Clear Temporal Relationship: TRALI
Transfusion-related acute lung injury (TRALI) is a subcategory of acute lung injury/acute respiratory distress syndrome (ALI/ARDS). According to a consensus definition, TRALI is defined as the occurrence of ALI within 6 h of transfusion of a blood product [10]. Any cell- or plasma-containing blood product can elicit a TRALI reaction and even small volumes can trigger the reaction. When other risk factors for ALI are concurrently present, one speaks of possible TRALI (Table 10.1).
Clinically, TRALI is indistinguishable from ALI due to other causes, characterized by pulmonary edema resulting in bilateral infiltrates on chest radiograph, with decreased oxygenation and subsequent dyspnea and tachypnea. Other clinical features are nonspecific and can include fever or hypothermia, hypotension as well as hypertension. Also laboratory testing in TRALI is not specific. The most prevalent symptom is a transient leukopenia, which occurs in 35–75 % [11], mostly occurring in patients after transfusion with an antibody containing blood product, which are thought to be due to neutrophil-specific antibodies. Also transient thrombocytopenia occurs .
Pathogenesis of TRALI: Two-Hit Mechanism
TRALI is mediated by the interaction of neutrophils with pulmonary endothelial cells. TRALI is thought to occur as a result of two independent hits. The first hit is the clinical condition of the patient at the time of the transfusion. An inflammatory reaction due to any cause attracts neutrophils to the pulmonary compartment. Primed neutrophils, trapped in the lung’s microvasculature, are functionally hyperactive. The second hit is the transfusion, which further activates the primed neutrophils in the lung vasculature of the recipient, resulting in endothelial damage, with ensuing pulmonary edema and extravasation of neutrophils. The second hit can be divided in antibody and non-antibody-mediated TRALI. There is near universal agreement that passive infusion of human neutrophil antibodies (HNA) or human leukocyte antibodies (HLA) directed against the recipients antigens can result in TRALI, called antibody-mediated TRALI. This is detailed below. However, many TRALI cases are reported in which no specific anti-neutrophil antibodies are detected [12, 13]. Also, the majority of recipients do not develop TRALI after infusion of antibodies, even when cognate antigen is present [14, 15]. Also, observational studies report associations between prolonged storage of blood products and ARDS in the critically ill [16, 17], although this was not confirmed in a recent randomized trial [18]. Thereby, there is also a non-antibody-mediated pathway in TRALI. The causative factor is presently not known. Data on the role of bioactive lipids (lysophosphatidylcholines, lysoPCs) or other neutrophil priming agents which have accumulated during blood storage are summarized below.
Pathogenesis of TRALI: Importance of Host Factors in the First Hit
The First Hit
Recent data point towards an important role for host factors in TRALI pathogenesis. In a study on risk factors for TRALI in a cohort of critically ill adults [19] as well as in critically ill children [20], around 90 % of the patients that confirmed to the diagnostic criteria for TRALI, also had a risk factor for ALI prior to onset of TRALI (possible TRALI). In the multivariate analysis, patient related risk factors were more important for the onset of TRALI than transfusion related risk factors, suggesting that development of a TRALI reaction depends more on host factors then on factors in the blood product. Of note, in a cohort of general TRALI patients as well as in cardiac surgery patients with TRALI, systemic levels of IL-6 and IL-8 were found to be elevated prior to the development of TRALI compared to controls [21, 22].
Specific Patient Risk Factors
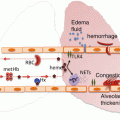
These are summarized in Fig. 10.1. The presence of mechanical ventilation predisposes to acquiring TRALI. This was shown in experimental models [23]. Also, the application of high peak airway pressures increases the risk for TRALI in patients [21]. Thereby, it seems likely that mechanical stretch of the lungs can prime pulmonary neutrophils. Given that an inflammatory condition can be a first hit, it is unsurprising that sepsis has repeatedly been shown to be an important risk factor for TRALI [17, 21]. Also specific surgical procedures seem a risk factor. The high incidence of TRALI in cardiac surgery is associated with duration of cardiopulmonary bypass, suggesting that this device may contribute to neutrophil priming. Also thoracic, vascular and transplant surgeries carry a high rate of perioperative TRALI, with an incidence of 2–3 %. Interestingly, obstetric and gynecologic surgeries are not risk factors [24]. Conditions in which patients typically receive multiple transfusions are also risk factors for TRALI, including hematologic malignancy, bleeding with liver failure and massive transfusion [17, 21]. In these settings, it is not clear whether these conditions are specific risk factors or merely reflect frequent exposure to blood products.
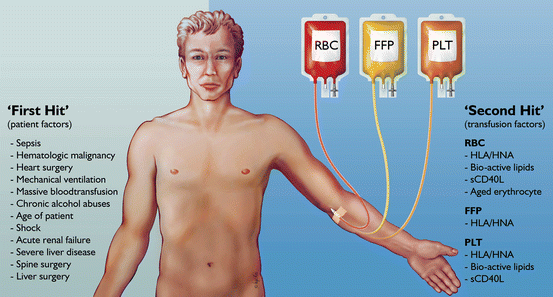

Fig. 10.1
Host and transfusion factors in the onset of transfusion-related acute lung injury (TRALI) . The first hit is the underlying condition of the patient resulting in priming of the pulmonary neutrophils. Risk factors which may function as a first hit are on the left side of the panel. The second hit is the blood transfusion resulting in activation of the endothelial cells and the primed pulmonary neutrophils resulting in capillary leakage, finally resulting in pulmonary oedema (right side of the panel). Some transfusion factors are independent of the type of blood product while others are specific for a type of blood product. HLA human leukocyte antibodies, HNA human neutrophil antibodies, RBCs red blood cells , FFP fresh frozen plasma
Pathogenesis of TRALI: Antibody Mediated TRALI
HLA and HNA Antibodies
All blood products have been implicated in TRALI. Antibody mediated TRALI is caused by the passive infusion of HLA and or HNA antibodies present in the plasma of the donor product. A minority of cases are caused by antibodies of the recipient reacting with the antigens of the infused leukocytes of the donor. However, since the implementation of a universal leukoreduced blood product program this form of antibody mediated TRALI has become irrelevant. High volume plasma products such as fresh frozen plasma (FFP) and platelet concentrates are at highest risk to induce TRALI. However any plasma containing product is able to induce TRALI, even with small amounts of residual plasma in RBCs products [25]. The involved antibodies in antibody mediated TRALI are HLA class I, HLA class II, and HNA antibodies (Table 10.2). The majority of reported TRALI reactions are based on antibodies present in the transfused blood products. Observational studies report that 14–26 % of the TRALI cases are caused by HLA class I antibodies, 0–46 % by HLA class II antibodies and 16–28 % by HNA antibodies [26–29]. Studies aimed at understanding which donors are at risk for developing antibodies showed that previously exposed donors such as multiparous donors have an increased risk of developing HLA antibodies. Screening of multiparous donors showed that due to sensitization during pregnancy up to 40 % developed antibodies [30]. Based on these findings a trial was performed among critically ill patients [31] in which 100 patients in need of plasma transfusion, were randomly assigned to receive plasma from a multiparous donor or from a control donor. This study showed that transfusion of plasma from multiparous donors was associated with decreased oxygen saturation and higher TNF-alpha concentrations than transfusion of control plasma. The strong relation between the presence of antibodies and TRALI resulted in the implementation of a male-only donor policy. Two recent meta-analyses showed that the implementation of these low risk TRALI donor policies for high volume plasma products has resulted in an approximately two-thirds reduction of TRALI cases [32, 33]. A remaining discussion is whether low risk TRALI donor policies should also be implemented for low volume plasma products such as RBCs.
Table 10.2
Host factors involved in the onset of antibody mediated TRALI
Antibody pathway | Involved host cells | Cofactors | Alternative pathway |
|---|---|---|---|
HLA class I | Platelet Neutrophil Monocyte | Complement | Direct on endothelium |
HLA Class II | Neutrophil Monocyte | ||
HNA | Neutrophil | Antibody fragments |
Downstream Host Response in Antibody Mediated TRALI
In the past decades, studies have clarified the role of host immune cells, endothelial cells, and cofactors in the onset of antibody mediated TRALI. Initially it was hypothesized that all antibodies (HLA class I, II and HNA) involved in the onset of antibody mediated TRALI induced neutrophil activation using a common pathway. Recent studies however showed that each antibody induces TRALI using separate pathways (Table 10.2). Most preclinical studies have been performed using HLA class I antibodies. The first in vivo mice model of TRALI used HLA class I antibodies [34]. Infusion of these antibodies resulted in severe pulmonary edema which made it a clinical relevant model. In this model depletion of neutrophils using vinblastine prevented the onset of TRALI . Also, depletion of platelets as well as aspirin, which prevents activation of platelets , protected mice for the onset of TRALI [35]. These studies suggest that both neutrophils and platelets are essential in the onset of TRALI. Another group, however, showed that HLA class I antibodies are able to induce TRALI in the absence of neutrophils and platelets using hydroxycarbamide and neuraminidase respectively [36]. It was suggested that complement and monocytes are key players in the onset of TRALI induced by HLA class I antibodies. Furthermore, from a TRALI case in a single lung-transplantation patient in which infusion of HLA class I antibodies resulted in TRALI only in the transplanted lung which expressed the cognate antigen and not in the native lung [37], it can be hypothesized that HLA class I antibodies are also able to directly activate and damage the endothelium.
The onset of antibody mediated TRALI through infusion of HLA class II antibodies has been studied less extensively. In vitro studies showed that HLA class II antibodies are able to activate monocytes resulting in damage to endothelial cells [38]. In an ex vivo animal model it was shown that activated monocytes are able to activate neutrophils which are hypothesized to be essential in the onset of TRALI induced by HLA class II antibodies [39].
The HNA antibodies are divided in several subtypes of which HNA-1, HNA-2, and HNA-3a are related to the most severe and even fatal TRALI reactions. HNA 3a antibodies have been shown to interact directly with the endothelial cells and cause lung injury, however neutrophils seem to be required for the onset of HNA induced lung injury [40]. Also HNA 3a antibody fragments can induce a mild form of lung injury. Of interest, HNA 3a fragments are not able to bind the Fcy-receptor, whereas the whole antibody can. This observation suggests a neutrophil Fcy-receptor dependent and independent pathway in the onset of HNA induced TRALI.
To summarize, antibody mediated TRALI is caused by the passive infusion of HLA class I, II and HNA antibodies reacting with the cognate antigen of the recipient. The pathways involved in the onset of antibody mediated TRALI differ between the involved antibodies. Studies suggest that besides neutrophils, also monocytes, platelets , endothelial cells and complement are essential in the onset of antibody mediated TRALI .
Pathogenesis of TRALI: Non-antibody-mediated TRALI
Soluble Mediators
Bioactive lipids accumulating during storage of red blood cells or platelets have been implicated in TRALI. The neutral (nonpolar) lipids are only found in RBCs, whereas lysophosphatidylcholines (LysoPCs) are thought to be platelet derived. In vitro, neutrophils can be activated by LysoPCs. LysoPCs isolated from aged blood also caused lung injury in a two-hit animal TRALI model [41] and LysoPCs have been implicated in a series of ten patients with TRALI [42]. On the other hand, other study groups could not confirm a role for lipids using two-hit TRALI animal models. Clinical studies report conflicting results on the role of LysoPCs. It is hypothesized that the association between LysoPCs and TRALI merely reflects a higher total volume of products transfused and not an increased level of lysoPCs per product due to accumulation during storage [17, 43]. These differences may relate to different storage procedures. Nonpolar lipids from RBCs caused acute lung injury in a two-hit animal model [44], which could partly be prevented by filtration of donor blood before storage [4]. The role of nonpolar lipids in human TRALI has not been explored yet.
Besides lipids , soluble CD40L has been implicated in non-antibody-mediated TRALI [45]. Soluble CD40L is produced by platelets . It activates macrophages and elicits the production of pro-inflammatory cytokines. Increased levels of sCD40L in stored platelets has been associated with transfusion reactions and increased respiratory burst in granulocytes. In animals developing TRALI, the level of sCD40L was modestly increased in the supernatant of stored RBCs or platelets compared to controls [6, 46]. Also, sCD40L levels were increased in samples of platelet transfusions implicated in TRALI reactions compared to transfused patients not developing TRALI [45]. On the other hand however, blocking of CD40L expression did not modulate TRALI in an animal model [47] and in a cohort of cardiac surgery patients with TRALI, sCD40L did not differ from transfused controls [48]. Even though sCD40L is a potent mediator in inflammation, a majority of studies suggest only a minor role for sCD40L in TRALI.
Cellular Changes
Red blood cells undergo changes during storage, among others decreased deformability, depletion of 2,3 diphosphoglycerate (2,3-DPG) and reductions in concentrations of nitric oxide. During storage, human RBCs also lose Duffy antigen expression and chemokine scavenging function. In a two-hit mouse model, transfusion of stored RBCs derived from Duffy-antigen knockout resulted in increased lung injury compared to transfusion of Duffy wild-type red blood cells , implicating a key role for the Duffy-antigen in TRALI [49]. Also platelets undergo changes when stored, including shrinking and plasma membrane vesiculation. In TRALI, platelet aggregation is found in histopathologic sections of the lung [35]. Whether aging of platelets plays a role is not clear however. Aged platelets , but not the supernatant of a product treated with ultraviolet, caused acute lung injury in two-hit murine models [50]. However, a recent study did not replicate these findings [51].
Downstream Host Response in Non-antibody-mediated TRALI
In vitro, stored RBC and stored platelets can prime fMLP-induced activation of the respiratory burst in human neutrophils compared to fresh products [46, 52]. Non-antibody-mediated TRALI caused by transfusion of stored RBCs or platelets in a two-hit animal TRALI model is characterized by increased pulmonary inflammation, including elevated levels of pro-inflammatory cytokines IL-6 and chemokine CINC3, as well as increased coagulation and impaired fibrinolysis compared to animals receiving fresh products. Also there is systemic inflammation [2, 6, 7]. Clinical studies on TRALI pathogenesis cannot differentiate between the antibody and non-antibody-mediated TRALI, as no specific diagnostic test exists to distinguish the two causes. In cardiac surgery patients with possible TRALI, pulmonary neutrophil influx is present and pulmonary levels of IL-6, IL-8, IL-1, and elastase–α1-antitrypsin complexes (a marker of neutrophil activation) are elevated during a TRALI reaction. In addition, pulmonary thrombin–antithrombin complexes and plasminogen activator inhibitor-1 were increased in the bronchoalveolar lavage fluid with a concomitant decrease in plasminogen activator activity percentage, indicating enhanced coagulation with impaired thrombolysis [22]. Also, in TRALI patients, levels of biomarkers for neutrophil extracellular traps (NETs) were increased compared to healthy subjects. As implicated blood did not contain excessive NET biomarkers, NETs supposedly were formed in the recipients after transfusion, suggestive of neutrophil activation [53]. A wide range of other mediators have been implicated in TRALI, the pathogenesis of which is summarized in Fig. 10.2. Of note, a distinction between causes of TRALI is not made.
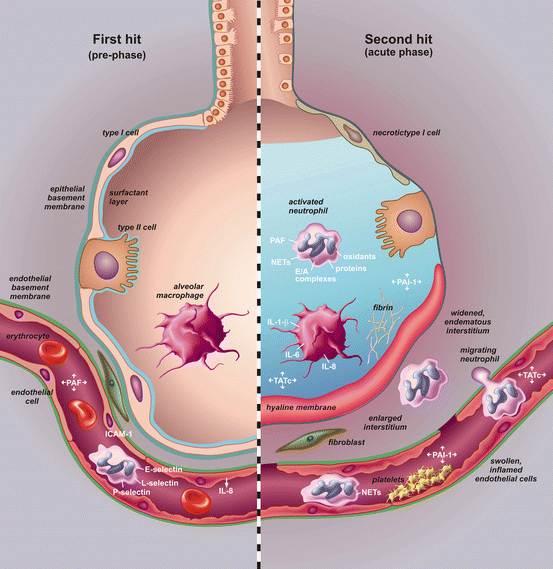

Fig. 10.2
Pathogenesis of transfusion-related acute lung injury (TRALI). In the pre-phase of the syndrome (left-hand side), there is a first hit which is mainly a systemic hit. Neutrophils are attracted to the lung by release of cytokines and chemokines from up-regulated lung endothelium. Loose binding by L-selectin takes place. Firm adhesion is realized by E-selectin and platelet-derived P-selectin and intra cellular adhesion molecules (ICAM-1). In the acute phase of the syndrome (right-hand side), there is a second hit resulting in inflammation in the pulmonary compartment. Neutrophils are shown adhering to the injured capillary endothelium and marginating through the interstitium into the air space, which is filled with protein-rich oedema fluid. In the air space, there is secretion of cytokines, interleukin-1, -6, and 8, (IL-1, IL-6, IL-8), which act locally to stimulate chemotaxis and activate neutrophils which results in elastase–α(1)-antitrypsin (EA) complex formation. Neutrophils can release oxidants, proteases, and other pro-inflammatory molecules, such as platelet-activating factor (PAF) and form neutrophil extracellular traps (NETs). Furthermore there is activation of the coagulation system illustrated by an increase in thrombin-antithrombin complexes (TATc) and decrease of the fibrinolysis system illustrated by a decrease in plasminogen activator activity (PAA%). The net result is influx of protein-rich oedema fluid into the alveolus with inactivation of surfactant
Pathogenesis of TRALI : Threshold Model
Previously, it was thought that TRALI due to antibodies did not require the presence of a priming first hit, as cases have been described in which relatively healthy recipients develop antibody mediated TRALI. However, in an experimental mouse model of TRALI, infusion of MHC-I antibodies in mice with the cognate antigen induces a TRALI reaction in the presence, but not in the absence of priming factors. A threshold model has been suggested which describes the relation between the presence of host factors (“first hit”) and factors in the blood product (“second hit”). In this model, a threshold must be overcome to induce a TRALI reaction [54]. It is proposed that transfusion of high titers of antibodies resulting in a strong antibody-mediated response does not “require” an inflammatory condition that primes neutrophils as a first hit, but can cause TRALI in a healthy recipient. Conversely, it is possible that activating factors in the transfusion are not strong enough to overcome the threshold when priming status is too low. This would explain why TRALI does not develop in transfused patient even when an antibody–antigen match is present. However, in a patient with a predisposing factor, transfusion of mediators with low neutrophil-activating capacity is sufficient to overcome the threshold to induce a TRALI reaction.
Transfusion and ARDS : The Association
The most striking suggestion of a relationship between transfusion and ARDS comes from a trial in critically ill and trauma patients, in which a liberal transfusion trigger was associated with the development of ARDS (OR 1.5 (CI 1.0–2.5) compared to a restrictive transfusion trigger [55]. However, a difference in pulmonary complications was not noted in a trial comparing liberal vs. restrictive triggers in patients with gastrointestinal bleeding [56]. The concept that an inflammatory predisposition may render the host susceptible to TRALI suggests that critical care or injury is a general host factor risk. Therefore, is TRALI a distinct phenomenon or the result of a synergy between transfusion and other mechanisms that cause ALI? The time frame in the conventional definition of TRALI is 6 h, which is based on expert opinion, probably based on observations of patient cases. Obviously however, a clock does not start ticking when a transfusion is started. The term “delayed TRALI” has been coined for the critically ill when ALI occurs 6–72 h after a blood transfusion [57]. Using this time frame, transfusion increases the risk for the development of the ALI 6–72 h after the transfusion in a cohort of critically ill or injured patients with an OR of 2.1 (CI 1.7–2.5). The question can be raised regarding what distinction exists, if any, between transfusion resulting in lung injury within 6 h and lung injury associated with transfusion.
Transfusion and ARDS : Is There a Two-Hit Mechanism?
The association between transfusion and ARDS is particularly clear in the critically ill, trauma, and cardiac surgery patients. In unselected critically ill populations, incidence rates of ARDS associated with transfusion are as high as 25 % [57]. In transfused trauma, incidence rates of ARDS are around 5–10 % and 4–12 % in transfused cardiac surgery. In contrast, cancer patients, who are also frequently exposed to transfusion, are not at increased risk of ARDS following transfusion as compared to other patient populations [58]. These differences in incidence suggest that an underlying inflammatory condition contributes to the risk of developing ARDS following transfusion. This is compatible with the two-hit hypothesis.
Transfusion and ARDS : Risk Factors
Patient Risk Factors
Requirement of massive transfusion has been long known as a risk factor for ARDS [59, 60]. The association between transfusion and ARDS is dose dependent. A meta analysis showed that the odds of developing ARDS following trauma or surgery are increased with each additional red blood cell unit transfused [61]. Another clear patient related risk factor is mechanical ventilation. In ventilated patients, ARDS following transfusion is reported to be as high as 30 % [62]. In a study in critically ill children, mechanical ventilation was present in all children who developed ARDS following transfusion [20]. Given that settings of the ventilator can influence the occurrence of ARDS [63], it seems clear that mechanical ventilation contributes to the risk of developing ARDS following transfusion. In trauma patients, besides massive transfusion, also shock and thoracic trauma are risk factors for ARDS following transfusion [61, 64].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree