Over the last decade, transarterial therapies have gained worldwide acceptance as standard of care for inoperable primary liver cancer. Survival times after transarterial chemoembolization (TACE) continue to improve as the technique and selection criteria are refined. Transarterial treatments, frequently provided in an outpatient setting, are now safely and effectively being applied to patients with even advanced malignancy or partially decompensated cirrhosis. In the coming years, newer transarterial therapies such as radiation segmentectomy, boosted-transarterial radioembolzation, combined TACE-ablation, TACE-portal vein embolization, and transarterial infusion of cancer-specific metabolic inhibitors promise to continue improving survival and quality of life.
Key points
- •
Choosing the appropriate transarterial modality to aggressively treat malignancy while preserving function and lower portal pressures is central to patient selection for transarterial therapy.
- •
Current transarterial techniques have been proved safe and effective in advanced hepatocellular carcinoma and Child B cirrhosis. The posttreatment prognosis for Child A patients continues to improve.
- •
Many modern transarterial therapies cause minimal postembolization syndrome and are therefore routinely provided as outpatient procedures.
- •
Meticulous imaging follow-up and retreatment of new or recurrent lesions is imperative for ensuring maximum survival after any transarterial therapy.
- •
Current transarterial treatment options are safer and more effective than treatments of a decade ago. New techniques will continue this trend via patient-specific therapies in the future.
Introduction
Transarterial therapy for hepatocellular carcinoma (HCC) was first described in the medical literature in the late 1970s. In the 1980s, several reports discussed the feasibility of combining embolic and chemotherapeutic agents. In 2002, 2 separate randomized controlled trials (RCTs) each showed longer survival by patients with HCC receiving transcatheter arterial chemoembolization (TACE) compared with those receiving best supportive care (BSC). Since these two RCTs, novel transarterial techniques have continued to be developed, and the management of patients with primary liver cancer has also continued to evolve.
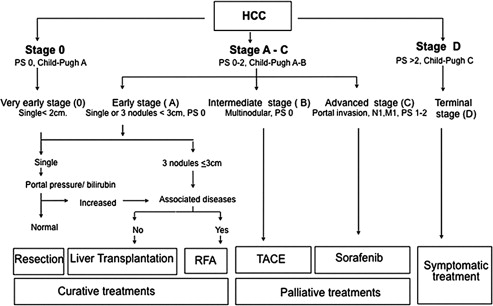
The treatment of patients with HCC has become standardized as a result of several international guidelines. Fig. 1 describes a widely used algorithm that serves as the dominant general guideline for whether a patient undergoes transplantation, surgical resection, ablation, transarterial treatment, or systemic therapy. Because it is still a minority of patients with primary liver cancer who can be cured with resection or transplantation, percutaneous and transarterial interventional techniques remain essential in the management of patients with HCC and intrahepatic cholangiocarcinoma (ICC). Percutaneous techniques are addressed elsewhere in this issue by Clary and colleagues. This article describes current transarterial therapies in the management of primary liver cancers.
Introduction
Transarterial therapy for hepatocellular carcinoma (HCC) was first described in the medical literature in the late 1970s. In the 1980s, several reports discussed the feasibility of combining embolic and chemotherapeutic agents. In 2002, 2 separate randomized controlled trials (RCTs) each showed longer survival by patients with HCC receiving transcatheter arterial chemoembolization (TACE) compared with those receiving best supportive care (BSC). Since these two RCTs, novel transarterial techniques have continued to be developed, and the management of patients with primary liver cancer has also continued to evolve.
The treatment of patients with HCC has become standardized as a result of several international guidelines. Fig. 1 describes a widely used algorithm that serves as the dominant general guideline for whether a patient undergoes transplantation, surgical resection, ablation, transarterial treatment, or systemic therapy. Because it is still a minority of patients with primary liver cancer who can be cured with resection or transplantation, percutaneous and transarterial interventional techniques remain essential in the management of patients with HCC and intrahepatic cholangiocarcinoma (ICC). Percutaneous techniques are addressed elsewhere in this issue by Clary and colleagues. This article describes current transarterial therapies in the management of primary liver cancers.
Transarterial therapeutic options
Table 1 lists the 5 main categories of transarterial therapy used in current practice. Transarterial therapies are usually performed under moderate sedation with independent radiology nursing supervision for most patients, including pulse oximetry, cardiac monitoring, and blood pressure monitoring. When warranted by a patient’s comorbidities, procedures may be performed with light sedation or under deep sedation with anesthesiology assistance.
| Transarterial Technique | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|
| TACI | Intrahepatic chemotherapy, often suspended in oily contrast, without use of any other embolic | Absence of permanent embolic improves vessel patency for future transarterial treatment Low cost | Widely varying technique |
| cTACE, also called oily or Lipiodol TACE | Intrahepatic chemotherapy held in tumors by oily contrast, tumor ischemia by oil and additional temporary or permanent embolic material | Proved superior to supportive care by RCT Low cost | Widely varying technique Postembolization syndrome may require inpatient treatment |
| TAE also called bland embolization | Ischemic necrosis at the arteriolar level, typically with small (eg, 40–120 μm), permanent embolic material | Theoretically better side effect profile than cTACE Simplest transarterial technique, with potential for simple standardization Low cost | Less durable than DEB-TACE Potential for pulmonary emboli from small-particle treatment of large tumors near diaphragm Postembolization syndrome may require inpatient treatment |
| DEB-TACE | Intrahepatic chemotherapy slowly eluted by embolic microspheres | Less systemic chemotherapy leakage from liver than cTACE, with resultant lower systemic toxicity Can be less embolic than TAE or cTACE, with resultant lower ischemic toxicity to liver More standardized treatment technique than TACI or cTACE | Greater potency of individual microspheres requires possible prophylactic coiling of cystic or hepaticoenteric collateral arteries Postembolization syndrome may require inpatient treatment Greater cost than cTACE |
| Y90 radioembolization (TARE/SIRT) | Arterially directed intratumoral brachytherapy | Some data to support superior HCC downstaging vs TACE; role in PVT Radiation segmentectomy and lobectomy modifications of TARE may allow greater safety and effectiveness Fewer treatment sessions required than with other transarterial techniques Minimally embolic treatment allows safe lobar therapy despite PVT Less severe postembolization syndrome allows routine outpatient treatment | Each treatment requires coordination between interventional radiology; radiation safety; nuclear medicine; and, frequently, radiation oncology Meticulous angiographic technique required to avoid nontarget embolization Greatest per-treatment session cost of all transarterial techniques Small particle size may cause greater biliary toxicity than other transarterial techniques |
The wide availability of advanced cross-sectional imaging now frequently allows the interventionalist to forego aortic angiography, thus reducing x-ray exposure and contrast dose at the time of intervention. Focused sonographic examination in the interventional radiology suite often allows confirmation of hepatopetal portal flow, in many cases obviating routine superior mesenteric artery angiography.
Although conventional TACE (cTACE) has typically required inpatient admission for management of postprocedural pain, fever, and nausea, known as postembolization syndrome, many newer transarterial techniques cause less postembolization syndrome and are routinely performed as outpatient procedures.
cTACE
cTACE, the most widely practiced transarterial therapy, consists of filling the arterial supply of the target tumor or tumors with a mix of chemotherapy and embolic material. cTACE uses sterile iodinated poppy seed oil (Lipiodol Ultra-Fluide, Guerbet, Villepinte, France; previously also available as the Ethiodol brand) to create a viscous, highly radiopaque emulsion with a chemotherapeutic agent or agents that is infused into the tumoral arterial supply. This emulsion is typically either mixed with or followed by infusion of additional embolic material.
A variety of chemotherapeutic and embolic agents are currently in use in transarterial therapy for primary hepatic malignancy. Doxorubicin is currently the most commonly used chemotherapeutic in single-agent TACE in North America. Triple-agent TACE commonly uses a mix of cisplatin, doxorubicin (Adriamycin), and mitomycin C (CDM-TACE or CAM-TACE).
Spherical embolic material has generally replaced older, nonspherical embolic agents because of the availability of more tightly calibrated sizes and greater predictability of flow dynamics. Both temporary (eg, calibrated Gelfoam and starch microspheres) and permanent (eg, trisacryl gelatin [TAG] and spherical polyvinyl alcohol [PVA]) embolic agents are used, ranging in size from 40 to more than 1000 μm in diameter.
Transarterial Embolization
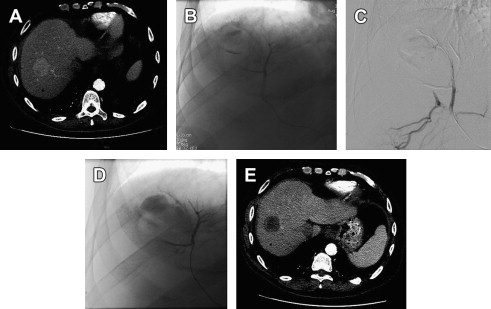
Although cTACE traditionally uses 100-μm to 500-μm diameter embolic, transarterial embolization without chemotherapy, also called bland embolization, as currently practiced more often makes use of smaller 50-μm PVA or 40-μm to 120-μm TAG microspheres in an effort to occlude the arteriolar tumoral blood supply while preserving patency of larger feeding arteries, allowing subsequent additional transarterial treatment. The primary procedural end point of both transarterial embolization (TAE) and TACE is the same: angiographic evidence of embolization (ie, stasis or near stasis) of arterial supply to the target tumors. However, the mechanisms of action differ, with bland embolization causing ischemia, whereas cTACE also relies on the chemotherapeutic effect for tumor necrosis. Fig. 2 shows a case of HCC treated with the modern small-particle TAE technique.
Drug-eluting Bead TACE
First reported in the treatment of metastatic colorectal cancer in 2006 and for HCC in 2007, drug-eluting bead (DEB) TACE is a modification of TACE in which a permanent embolic microsphere is soaked in a single chemotherapeutic agent in advance of the treatment session and then, once embolized transarterially, slowly elutes the agent within the tumor over a period of several days. Two products are currently available in the United States: LC Beads (Biocompatibles, BTG International Inc, West Conshohocken, PA) are PVA hydrogel microspheres, and QuadraSpheres (Merit Medical, South Jordan, UT) are hydrophilic microspheres consisting of sodium acrylate alcohol. For administration under fluoroscopic guidance, the chemotherapy-soaked microspheres are suspended in saline and aqueous iodinated contrast, usually without Lipiodol or any other additional embolic material.
Transarterial Chemoinfusion
Transarterial chemoinfusion (TACI), as the term is applied in the treatment of primary liver cancers, is typically defined as transarterial delivery of chemotherapy suspended in Lipiodol but without any other embolic material. Some investigators restrict the term TACI to exclude even Lipiodol. Others consider superselective TACE using a temporary embolic (eg, Gelfoam) to be TACI; however, these are in the minority. TACI, as it is commonly defined, is currently less commonly used in North America than TACE and TAE.
Transarterial Radioembolization
Transarterial radioembolization (TARE), also known as selective internal radiotherapy (SIRT) or radiomicrobrachytherapy (RMB), was originally described in 1986 as transarterial infusion of 131 I-labeled Lipiodol for inoperable HCC. Perhaps because of the greater regulatory requirements of open-source radiation therapy, development of TARE for liver cancer has been a more gradually evolving process. The first case series of yttrium-90 (Y90) TARE in humans was published in 1992 using resin microspheres in patients with metastatic colorectal cancer. Glass and resin radiomicrobrachytherapy devices are now commercially available in North America with which to perform TARE. The TheraSphere (glass microspheres) device was approved by the US Food and Drug Administration (FDA) in 2000 as a humanitarian use device for patients with unresectable HCC. SIR-Spheres (resin microspheres) were granted premarket FDA approval in 2002 for treatment of metastatic colorectal carcinoma in the liver.
TheraSphere Y90 microspheres (BTG International Inc, West Conshohocken, PA) are nonbiodegradable glass microspheres with Y90 as an integral constituent. TheraSphere range from 20 to 30 μm in diameter and have a specific gravity of 3.6 g/dL and a specific activity of 2500 Bq/sphere. A 3-GBq vial contains 1.2 × 10 6 microspheres (TheraSphere package insert). Specific doses are infused by ordering a predetermined-dose vial and coordinating the day and time of administration with published decay curves. Oversight by an institutional review board is required to administer TheraSphere.
SIR-Spheres (Sirtex Medical, Lane Cove, Australia) are resin microspheres onto which Y90 is bound. They range from 20 to 60 μm in diameter and have a specific gravity of 1.6 g/dL and a specific activity of 50 Bq/sphere. A 3-GBq vial contains 40 × 10 6 to 80 × 10 6 microspheres (SIR-Spheres package insert). SIR-Spheres arrive in a standard-dose vial on the day of treatment. The receiving institution’s radiopharmacist decants an appropriate volume of spheres to achieve the prescribed activity for treatment. Use of SIR-Spheres for HCC and ICC is off-label.
Characteristics of Y90 that facilitate its use in transarterial radioembolization are common to both devices. Y90 is a pure beta particle emitter that decays to stable zirconium-90 (Zr-90) with a half-life of 64.1 days. The average energy of beta emission is 0.9367 MeV, with mean and maximum soft tissue penetrations of 2.5 and 10 mm, respectively. TARE takes advantage of the tendency of many tumors to recruit more arterial supply than benign hepatocytes, even some tumors that appear hypovascular on contrast computed tomography (CT) or magnetic resonance imaging (MRI). There is therefore shunting of hepatic arterial flow toward tumors and preferential deposition of radiomicrospheres within the tumors and away from benign liver tissue.
Landmark clinical outcomes, key current data, and comparisons of modern transarterial techniques
Although the literature on transarterial therapies for ICC continues to grow, the majority of research into transarterial therapy for primary liver cancer has focused on HCC. Text in this section will focus on HCC therapies. A summary of recent trials investigating transarterial therapies for ICC is listed in Table 2 .
| Primary Author, Year | Study Type | Type(s) of Treatment | Number of Subjects | Mean or Median a of Treatment Sessions | Noncirrhotic or CP A | ECOG 0 (%) | ECOG 1 (%) | ECOG 2+ (%) | Prior Chemotherapy (%) | Prior External Beam Radiotherapy (%) | Prior Thermal Ablation (%) | Prior Surgical Resection (%) | Single Tumor (%) | Peripheral/No Ductal Invasion (%) | Unilobar Disease (%) | Extrahepatic (%) | PVT/Vascular Invasion (%) | Hypervascular Tumor (%) | Approx % Liver Volume Replaced by Tumor (<25%, <50%, <75%) | Treatment Response Imaging Criteria at 3 mo or First After Treatment | Objective Response (%), SD (%), PD (%) | Median OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliberti et al, 2008 | CC | Doxorubicin DEB-TACE vs systemic fluorouracil | 11/9 | 2.6 | — | — | — | — | b | b | b | b | — | — | — | — | — | — | — | RECIST | 91, 0, 0 | 13/7 |
| Gusani et al, 2008 | CS | cTACE: gemcitabine, cisplatin, oxaliplatin or gem-cis combo; all with TAG microspheres | 42 | 3.5 | — | — | — | — | — | — | — | — | — | 12 | — | 45 | — | — | — | RECIST | 0, 57, 43 | 9.1 |
| Ibrahim et al, 2008 | CS | Glass microspheres TARE | 24 | 2 | — | 42 | 50 | 8 | 29 | — | — | 4 c | 46 | 71 | 33 | 33 | 38 | — | 83, 13, 4 | World Health Organization; EASL | 27, 68, 5; 86, —, — d | 14.9 e |
| Kim et al, 2008 | CS | Cisplatin TACI (n = 13) or cisplatin, Lipiodol and Gelfoam cTACE (n = 36) | 49 | 3 | 82 | — | — | — | 4 f | 33 f | — | — | 29 | 90 | — | 51 | — | 73 | — | RECIST | 55, 31, 14 | 10 |
| Poggi et al, 2009 | CC | Systemic gem-ox vs systemic gem-ox + oxaliplatin DEB-TACE | 11/9 | 3.3 | 91/100 | — | — | 0 | — | — | — | 0/22 c | — | — | — | 0 | — | — | — | RECIST | 0/44, 73/56, 27/0 | 20/30 e |
| Saxena et al, 2010 | CS | Resin microspheres TARE | 25 | 1 | — | 60 | 28 | 12 | 72 | — | 8 | 40 | — | 60 | 20 | 48 | 0 | — | 40, 60, 0 | RECIST | 26, 48, 22 | 9.3 e |
| Kiefer et al, 2011 | CS | cTACE: cisplatin, doxorubicin and mitomycin C with PVA spheres | 62 | 2.7 | — | 89 | 10 | 1 | 29 | 3 | 5 | 11 | — | — | — | — | — | — | — | RECIST | 11, 64, 24 | 15 g |
| Park et al, 2011 | CC | cTACE: cisplatin, Lipiodol and Gelfoam vs BSC | 72/83 | 2.5 | — | 65/54 | 32/41 | 3/5 | 0/0 | 0/0 | 0/0 | 0/0 | 43/53 | — | 49/51 | 54/60 | — | 18 h /12 h | — | RECIST | 23/NA, 67/NA, 11/NA | 12.2/3.3 |
| Shen et al, 2011 | CC | Fluorouracil or carboplatin, epirubicin and hydroxycamptothecin TACI ± Lipiodol cTACE vs systemic therapy or BSC only | 53/72 | — | 60/61 | — | — | — | — | — | — | 100, 4 c /100, 4 c | 63/68 | 92/83 | — | 6/10 | — | — | — | — | — | 12/5 |
| Hoffman et al, 2012 | CS | Resin microspheres TARE | 33 | 1 | — | 52 | 21 | 27 | 82 | 3 | 6 | 36 | 30 | 36 | 36 | 24 | 0 | — | 76/24/0 | RECIST | 36, 52, 15 | 22 |
| Kuhlmann et al, 2012 | CC | Irinotecan DEB-TACE vs cTACE: mitomycin C + Gelfoam vs systemic oxaliplatin + gemcitabine | 26/10/31 | 1.6/1.4/NA | 100/100/100 | 62/70/65 | 35/30/32 | 4/0/3 | 19/20/0 | 4/10/3 | — | 4/0/23 | — | — | — | 42/40/90 | — | — | — | RECIST | 3/12.5/26, 42/12.5/45, 50/75/29 | 11.7/5.7/11.0 i |
| Rafi et al, 2013 | PCS | Resin microspheres TARE | 19 | 1.6 | — | 5 | 74 | 21 | 100 | 0 | 0 | 0 | 32 | — | 58 | 58 | — | — | — | RECIST | 11, 68, 21 | 11.5 |
| Vogl et al, 2012 | CS | cTACE: mito C, gem, mito-gem, or mito-cis gem-cis, + Lipiodol and starch spheres | 115 | 7.1 | 46 | — | — | — | — | — | — | — | 30 | — | 23 | 0 | 0 | 54 | — | RECIST | 9, 57, 33 | 13 |
| Hyder et al, 2013 | CS | cTACE (gem-cis, cis-dox-mito, gem, cis, or other); doxorubicin, cisplatin or other DEB-TACE; TAE; Y90 | 198 | — | — | 59 | 36 | 4 | 28 | — | 2 | 12 | 48 | 12 | — | 20 | 80 | — | — | mRECIST; EASL | 25, 62, 13; 34, 48, 18 | 13.2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree