Management of liver malignancies, both primary and metastatic, requires a host of treatment modalities when attempting to prolong survival. Although surgical resection and transplantation continue to offer the best chance for a cure, most patients are not amenable to these therapies because of their advanced disease at presentation. Taking advantage of the unique blood supply of the liver, transarterial chemoembolization has emerged as an alternative and effective therapy for unresectable tumors. In this article, the current role along with future perspectives of transarterial chemoembolization for hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and colorectal liver metastasis are discussed.
Key points
- •
Transarterial chemoembolization is a viable treatment option for unresectable liver tumors or tumors refractory to other therapies.
- •
Current chemoembolization techniques are being performed with greater safety and efficacy when compared with transarterial therapies performed in the past.
- •
Chemoembolization remains an acceptable approach for unresectable tumors with preserved liver function.
- •
Optimal chemoembolization strategies require further prospective studies.
Introduction
Over the past 2 decades, novel treatment modalities for primary and metastatic liver tumors have been developed. Although surgical resection continues to offer the best chance for long-term survival, up to 70% of patients with hepatic malignancies are deemed unresectable, thereby precluding many from a durable treatment approach. Liver transplantation remains an additional curative treatment; however, the scarcity of available organs continues to be the primary limitation of this modality. Instead, most patients rely on alternative therapies, often considered palliative, which attempt to maintain similar tenets to surgery: improve quality of life and prolong survival. Taking advantage of the unique dual blood supply of the liver, hepatic artery–based locoregional therapies have emerged as a treatment strategy for liver malignancies. In contrast to the normal liver parenchyma, which is supplied predominantly by the portal venous system, liver tumors are supplied almost exclusively by the hepatic arterial system, which provides the rationale for transarterial therapies.
First described in the late 1970s for the treatment of hepatocellular carcinoma (HCC), transarterial therapies continue to evolve and are essential for the management of HCC and other malignancies, which include intrahepatic cholangiocarcinoma (ICC) and colorectal liver metastases (CRLM). Although a variety of techniques such as bland embolization (TAE), transarterial chemoinfusion, and yttrium-90 radioembolization also use a transarterial approach, transarterial chemoembolization (TACE) remains the most widely performed and accepted locoregional therapy for inoperable liver malignancies. In this article, current evidence regarding the role of TACE and drug-eluting bead (DEB) TACE for HCC, ICC, and CRLM are examined and summarized.
Introduction
Over the past 2 decades, novel treatment modalities for primary and metastatic liver tumors have been developed. Although surgical resection continues to offer the best chance for long-term survival, up to 70% of patients with hepatic malignancies are deemed unresectable, thereby precluding many from a durable treatment approach. Liver transplantation remains an additional curative treatment; however, the scarcity of available organs continues to be the primary limitation of this modality. Instead, most patients rely on alternative therapies, often considered palliative, which attempt to maintain similar tenets to surgery: improve quality of life and prolong survival. Taking advantage of the unique dual blood supply of the liver, hepatic artery–based locoregional therapies have emerged as a treatment strategy for liver malignancies. In contrast to the normal liver parenchyma, which is supplied predominantly by the portal venous system, liver tumors are supplied almost exclusively by the hepatic arterial system, which provides the rationale for transarterial therapies.
First described in the late 1970s for the treatment of hepatocellular carcinoma (HCC), transarterial therapies continue to evolve and are essential for the management of HCC and other malignancies, which include intrahepatic cholangiocarcinoma (ICC) and colorectal liver metastases (CRLM). Although a variety of techniques such as bland embolization (TAE), transarterial chemoinfusion, and yttrium-90 radioembolization also use a transarterial approach, transarterial chemoembolization (TACE) remains the most widely performed and accepted locoregional therapy for inoperable liver malignancies. In this article, current evidence regarding the role of TACE and drug-eluting bead (DEB) TACE for HCC, ICC, and CRLM are examined and summarized.
Principles of transarterial chemoembolization
The advantage of TACE therapy rests in its ability to deliver high concentrations of cytotoxic agents to hypervascular liver tumors by selective disruption of feeding arteries and to minimize damage to the surrounding liver parenchyma. Combining chemotherapeutic drugs with embolic material results in a synergistic treatment effect; ischemic tumor necrosis and extended exposure of the tumor to the chemotherapeutic agent are the major treatment-related benefits of this approach. Moreover, the use of the embolizing agents facilitates lower systemic drug levels, thereby reducing toxicity.
Patient Selection
General indications for transarterial therapy for hepatic malignancies continue to evolve. Standard assessment of patients being considered for TACE generally involve evaluation of liver function, severity of portal hypertension, hepatic arterial anatomy, tumor size and distribution, comorbidities, and functional status. Although generally contraindicated in the setting of portal vein thrombosis, limited TACE therapy can be performed in the presence of hepatopetal flow via collateral vessels. Among the different hepatic malignancies, established guidelines currently exist for HCC. According to the American Association for Study of Liver Diseases (AASLD), TACE is recommended as a first line noncurative therapy for patients with large or multinodular tumors without vascular invasion, absence of extrahepatic disease, compensated liver disease (Child-Pugh A/B), and overall good performance status (Eastern Cooperative Oncology Group [ECOG] Performance Status 0). Although not in formal guidelines, additional variables described as contraindications to TACE have included age greater than 70/80 years, bilirubin levels greater than 3 mg/dL, tumor size 10 cm or larger, bile duct occlusion or incompetent papilla secondary to surgical manipulation, and greater than 50% replacement of the liver by tumor. Technical contraindications include untreatable arteriovenous fistula. TACE therapy necessitates prerequisites for the treatment of other unresectable liver malignancies similar to those indications for HCC. However, TACE has also been applied as a treatment adjunct in other clinical settings. Additional uses for TACE have included preoperative neoadjuvant therapy, tumor downstaging for potential resection/liver transplantation, and as salvage therapy for chemorefractory tumors.
Transarterial Chemoembolization
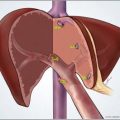
TACE remains the most widely performed transarterial therapy. Traditionally performed under moderate sedation, the procedure consists of selectively delivering via catheter injection a mix of a chemotherapeutic agent and embolic material to the tumor arterial supply ( Fig. 1 ). A variety of cytotoxic agents have been used. However, doxorubicin, cisplatin, epirubicin, mitoxantrone, mitomycin, and SMANCS (styrene maleic acid neocarzinostatin) remain the most common single agents for TACE. A triple-agent TACE regimen consisting of cisplatin, doxorubicin, and mitomycin C has also been described.
Delivery of the cytotoxic agent(s) to the tumor is aided by the use of Lipiodol (iodized oil, Guerbet, France) an oily contrast medium, which serves as a vehicle to carry and localize the drug inside the tumor. This procedure is generally followed by mechanical embolization by either spherical or nonspherical embolic agents. Although temporary, Gelfoam particles are the most commonly used embolizing agent. Other materials used to achieve arterial obstruction include polyvinyl alcohol (PVA), starch microspheres, trisacryl gelatin, or metallic coils. Successful embolization is usually defined as stasis in either the second-order or third-order lobar hepatic artery branches.
Drug-Eluting Bead Transarterial Chemoembolization
Similar to conventional TACE therapies, DEB TACE is an alternative technique that consists of highly absorbent PVA microspheres that have been modified with sulfonate groups, enabling active sequestering of cytotoxic compounds in their salt form when mixed together. After transarterial embolization (TAE), the DEB release the cytotoxic agent in a controlled fashion over several days. The most common cytotoxic agents used with the DEB platform include doxorubicin, oxaliplatin, and irinotecan. With DEB-TACE, studies have reported more favorable pharmacokinetics along with reduced systemic toxicity when compared with traditional TACE.
Assessment of Response to Therapy
Although the primary end point of cancer research and therapy development is overall survival, time to progression and tumor response continue to serve as important surrogate measures of treatment efficacy. The World Health Organization criteria and Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were originally designed to evaluate tumor response to chemotherapeutic agents. These measures were subsequently applied to assess locoregional therapies; however, the results were misleading because of the inability of the guidelines to assess tumor viability beyond anatomic size. In 2008, amendments to the RECIST guidelines, referred to as the modified RECIST assessment (mRECIST), included tumor enhancement on arterial phase during contrast-enhanced radiologic imaging as an additional criterion to assess the extent of tumor response to therapy.
According to the recent guidelines issued by the European Association for the Study of the Liver and the European Organization for Research and Treatment of Cancer, response to therapy should be based on contrast-enhanced radiologic imaging using mRECIST criteria ( Fig. 2 ). Furthermore, it is recommended that the initial radiographic assessment after therapy be performed 4 weeks after initial treatment. The direct tumor necrosis caused by TACE and DEB-TACE can be accurately characterized by mRECIST criteria and have been validated by recent studies showing its correlation with survival outcomes.
Toxicity/Complications
The overall rate and severity of treatment-related complications are variable for TACE and DEB-TACE therapies. Postembolization syndrome, which consists of transient abdominal pain, nausea, fever, fatigue, and increased hepatic transaminases, occurs most commonly (60%–80% of patients); however, it is not considered a complication but an expected side effect of the procedure. In contrast, major complications occur in up to 10% of patients, with 30-day mortality ranging from 2% to 4% for TACE. Development of hepatic abscesses and biliary sclerosis is the most common major complication. Other complications include liver failure, biloma, ischemic cholecystitits, gastrointestinal hemorrhage/ulceration, vascular injury, and pulmonary embolism, but these are less common (<1%).
Although the toxicity profile for DEB-TACE and TACE are similar, drug-related adverse events have been reported less often with DEB-TACE. In a recent series by Malagari and colleagues, complications associated with doxorubicin DEB-TACE (DEBDOX) therapy were evaluated in 237 patients. Using the National Cancer Institute Common Terminology Criteria for Adverse Events, grade 4 and 5 complications occurred in 5.48% and 1.26% of the cohort, respectively. Moreover, there were no periprocedural deaths, and the 30-day mortality was only 1.26%. Despite being a newer and evolving technology, the safety profile associated with DEB-TACE remains promising.
Hepatocellular carcinoma
Currently the third leading cause of cancer-related mortality worldwide, HCC remains a complex disease, which continues to pose challenges to the medical community. Factors leading to the development of HCC are well established (eg, viral hepatitis, alcohol consumption, and metabolic disorders, including nonalcoholic steatohepatitis); however, attempts at identifying early stage disease through the implementation of surveillance programs have resulted in suboptimal results. As a result, curative treatments (surgical resection, transplantation, or image-guided ablation), which are generally limited to early stage disease, are available to only a small percentage of patients with newly diagnosed HCC. Up to 70% of patients present with intermediate or advanced disease (Barcelona Clinic Liver Cancer (BCLC) classification: B and C, respectively), limiting the available treatment options.
Transarterial Chemoembolization for Hepatocellular Carcinoma
TACE therapy is the standard of care for intermediate stage (BCLC-B), multifocal HCC, without vascular invasion, or extrahepatic disease. However, initial studies evaluating the role of TACE compared with conservative management resulted in equivocal outcomes ( Table 1 ). It was not until 2002, when 2 seminal randomized controlled trials (RCTs) were published, that significant improvement in overall survival in patients with HCC treated with TACE was shown. In the study by Llovet and colleagues, 112 patients with unresectable HCC and compensated liver disease (Child-Pugh class A/B) were randomized to receive either doxorubicin-based TACE or conservative management only (symptom management). The investigators reported that patients who received TACE had a significant improvement in survival rates at 1 and 2 years (82% and 63%, respectively) compared with those who underwent conservative treatment (63% and 27%, P = .009). Moreover, in multivariate models, treatment allocation was independently associated with survival (odds ratio [OR] 0.45; 95% confidence interval [CI] 0.25–0.81; P = .02). Similarly, improved survival after cisplatin-based TACE compared with symptomatic treatment of patients with unresectable HCC was reported by Lo and colleagues. A total of 40 patients underwent TACE and experienced significant improvement in survival at 1 and 2 years (57% and 31%, respectively) compared with the control group (32% and 11%, P = .002). Although both studies reported a benefit after TACE, the relative superior survival in the trial by Llovet and colleagues compared with the trial by Lo and colleagues has been attributed to their strict enrollment criteria. Most patients recruited by Llovet and colleagues had compensated liver disease (70% Child-Pugh class A), with an overall good performance status (80% ECOG performance status 0), which likely contributed to the 27% 2-year survival experience by patients in the untreated control group. In contrast, broader enrollment criteria by Lo and colleagues resulted in patients with advanced disease being included in the cohort (57% with ECOG performance status 1/2/3, 27% with limited portal vein involvement). Despite the variable outcomes reported by the 2 studies, these trials were the first to show the potential survival benefit achieved with TACE and were pivotal in how patients with unresectable HCC were treated.
| Author, Year | No. of Patients | Study Design | Treatment Regimen | Median Survival (mo) | Overall Survival | ||
|---|---|---|---|---|---|---|---|
| 1 y (%) | 2 y (%) | 3 y (%) | |||||
| TACE | |||||||
| Pelletier et al, 1990 | 42 | RCT | Doxorubicin | NR | 24 | NR | NR |
| Trinchet et al, 1995 | 96 | RCT | Cisplatin | NR | 62 | 37.8 | NR |
| Llovet et al, 2002 | 112 | RCT | Doxorubicin | 28.7 a | 82 | 63 | 29 |
| Lo et al, 2002 | 80 | RCT | Cisplatin | NR | 57 | 31 | 26 |
| Brown et al, 2008 | 209 | RC | Cisplatin, Doxorubicin, mitomycin C | 15.5 | NR | NR | NR |
| Georgiades et al, 2006 | 172 | PC | Cisplatin, doxorubicin, mitomycin C | 18.3 | NR | NR | NR |
| Sahara et al, 2012 | 51 | RCT | Epirubicin vs | 21 | 85 | 76 | NR |
| ECMF | 19 | 95 | 65 | ||||
| DEB-TACE | |||||||
| Burrel et al, 2012 | 104 | RC | Doxorubicin | 48.6 | 89.9 | 66.3 | 54.2 |
| Malagari et al, 2013 | 45 | RC | Doxorubicin | NR | NR | NR | 62.2 b |
Multiple prospective and retrospective trials have since been published that have supported the role of TACE for HCC treatment. In a large retrospective analysis, Brown and colleagues recently reported their experience with TACE over a 15-year period. Of the 209 patients included in the study, the investigators reported a median overall survival of 15.5 months. In addition, the efficacy of TACE has been further bolstered by 2 meta-analyses. In 2003, a study by Llovet and Bruix evaluated 14 randomized clinical trials comparing TACE/TAE with either best supportive care or tamoxifen therapy (control). When compared with the control group, TACE/TAE resulted in a significant improvement in 2-year survival (41% vs 27%, OR 0.53; 95% CI 0.32–0.89; P = .17). Moreover, sensitivity analysis comparing TACE with best supportive care showed a significant benefit favoring TACE (OR 0.42; 95% CI 0.20–0.88). A second meta-analysis conducted by Marelli and colleagues reported similar results showing a significant decrease in mortality favoring TACE/TAE compared with nonoperative management (OR 0.705; 95% C: 0.499–0.994; P = .0026). As a result of these investigations, TACE has become the standard of care for patients with intermediate stage HCC (BCLC-B).
Although TACE has been established in the treatment algorithm for HCC, information regarding the optimal chemotherapeutic agent(s) remains equivocal. Multiple studies have failed to show a significant difference in survival when comparing doxorubicin with either epirubicin or cisplatin. In addition, a recent RCT of 63 patients comparing epirubicin versus multiagent (epirubicin, cisplatin, mitomycin C, and 5-fluorouracil) TACE was unable to identify a survival benefit between either regimen. In contrast, a retrospective study evaluating patients with HCC treated with doxorubicin or cisplatin/doxorubicin/mitomycin C (CDM), reported that patients treated with CDM-TACE experienced a higher tumor response rate and longer interval in progression-free survival. As a result, the variability in chemotherapy agent–related outcomes has resulted in a lack of uniform approach regarding the optimal type of cytotoxic therapy for TACE.
Drug-Eluting Bead Transarterial Chemoembolization for Hepatocellular Carcinoma
Compared with TACE, DEB-TACE is an emerging innovation in chemoembolization techniques. In earlier clinical trials, DEB-TACE was shown to maintain a favorable pharmacokinetic profile along with a low peak plasma concentration, which resulted in reduced rates of treatment-related toxicity. This finding subsequently led to the execution of the phase 2 randomized control study, PRECISION V, which compared the safety and efficacy of doxorubicin DEB-TACE with conventional TACE. Although the study was unable to show an overall survival difference or objective response between treatment arms in the collective cohort, subgroup analysis of patients with Child-Pugh B, ECOG 1, bilobar disease treated with DEB-TACE experienced a significant increase in objective response rate compared with TACE ( P = .038). Moreover, the DEB-TACE cohort versus the conventional TACE cohort reported a significant reduction in serious liver toxicity, resulting in improved patient tolerance to treatment ( P <.001).
Multiple retrospective studies have since been conducted that have supported the role of DEB-TACE for the treatment of HCC. In 2012, Burrel and colleagues evaluated the survival of patients with intermediate stage, unresectable HCC (BCLC-A/B) treated with DEB-TACE. Of the 104 patients treated, median and 5-year overall survival was 48.6 months and 38.3%, respectively, which was double the survival expectancy previously reported. Likewise, a smaller retrospective study conducted by Malagari and colleagues reported a 5-year overall survival of 62.2% among patients with intermediate stage HCC treated with DEB-TACE. Therefore, the improved treatment-related toxicity profile along with similar, or perhaps superior, survival benefit, suggest DEB-TACE as a better approach compared with conventional TACE for HCC treatment.
Combination Strategies
A common challenge faced by all TACE regimens is the durability of treatment. High rates of tumor recurrence or disease progression continue to limit the overall efficacy of TACE. The 2002 RCT conducted by Llovet and colleagues reported a sustained objective response of greater than 6 months after TACE in only 35% of their cohort. A potential cause of the high incidence of tumor recurrence that has been proposed relates to the derangement of the tumor microenvironment after TACE. It has been well documented that the hypoxia caused by TACE results in upregulation of inducible factor 1α, which in turn increases expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor, leading to tumor angiogenesis. As a result, there has been recent enthusiasm toward combining antiangiogenic targeted agents with TACE in attempts to improve the overall efficacy of therapeutic strategies.
Sorafenib, a multikinase inhibitor that inhibits angiogenesis by targeting the VEGF receptor 2, has been shown to improve overall survival (10.7 months) compared with placebo for advanced stage HCC. In 2011, the first of several phase 2 trials was published, which sought to evaluate the safety and efficacy of combining DEB-TACE with sorafenib for patients with advanced HCC. Although most patients in the series experienced at least 1 grade 3 to 4 toxicity, most toxicities were minor (grade 1–2, 83% vs grade 3–4, 17%). Moreover, preliminary efficacy data were promising, with a 95% reported disease control rate by RECIST criteria.
Multiple retrospective and phase 2/3 trials combining TACE with sorafenib have since been conducted, with mixed results. As a result, a recent meta-analysis was performed by Liu and colleagues, which sought to assess the safety and efficacy of the combination therapy. In total, 17 studies were included in the analysis, of which only 3 were RCTs. The investigators reported that time to progression was improved when TACE was combined with sorafenib (hazard ratio [HR] 0.76; 95% CI 0.66–0.89; P <.001) when compared with TACE alone. However, overall survival was not improved with combination therapy (HR 0.81; 95% CI 0.65–1.01; P = .061). Although the study was unable to show a survival benefit when sorafenib was combined with TACE, there was a wide variation in both treatment protocol and inclusion criteria among trials, which limited the ability of the study to fully assess the survival benefit of a combined approach. Despite the equivocal results surrounding the current literature regarding combined sorafenib and TACE therapy, well-designed phase 3 studies are ongoing and it is hoped will bring solace to this ongoing debate.
Expanding the Treatment Pool/Future Directions
According to the guidelines by the AASLD, HCC lesions with concomitant portal vein thrombosis are a contraindication to TACE. However, in recent years, attempts to expand the treatment pool have resulted in studies evaluating TACE in the setting of portal thrombus. In a single-institution prospective study by Georgiades and colleagues, 32 consecutive patients with unresectable HCC and portal vein thrombus were treated with TACE. The study reported an acceptable safety profile along with survival outcomes superior to historical best supportive care controls. The investigators concluded that in appropriately selected patients, portal thrombus should not be an absolute contraindication for TACE.
Understanding that resection and transplantation offer the best chance for long-term survival, TACE has also been used as a treatment adjunct for these modalities. A prospective study conducted by Luo and colleagues evaluated TACE in a neoadjuvant setting before resection. A total of 168 patients with large (>5 cm) or multinodular resectable HCC tumors received either TACE plus resection or resection alone. Patients who responded to TACE and subsequently underwent resection experienced 1-year, 3-year, and 5-year overall survival rates of 92%, 67%, and 50%, respectively. These results were significantly higher than the survival rates experienced by the resection alone cohort, suggesting that a combined approach is more effective in patients with large, or multifocal, resectable HCC tumors.
In addition, TACE has been used as a technique to downstage tumors to allow for transplantation. A case series in 2008 reported that 23.7% of patients in the study cohort were able to be successfully downstaged by TACE to qualify for liver transplantation under the Milan criteria. At a median follow-up of 19.6 months, 94.1% of patients who underwent successful liver transplantation were alive. The investigators reported that patients successfully downstaged with TACE can achieve similar survival outcomes to American Joint Commission on Cancer stage II patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree