Keywords
DTA-H19 plasmid, H19, oncofetal, cancer, transcriptional targeting, diphtheria toxin, tumor-specific promoter, hypoxia
Introduction
Reviewing brochures on the conventional cancer drugs in use today is undoubtedly a horrible and terrifying event. Seldom is any vital system in the body unaffected by a cancer drug, and sometimes they are seriously affected. Lack of tumor specificity is mostly responsible for these adverse events, which cause the therapeutic dose to be greatly limited. With few exceptions, these conventional therapeutic regimens also have narrow therapeutic windows; this is especially the case for locally advanced malignancies and for those that metastasize. Chemotherapy has resulted in a very modest survival gain and has not demonstrated a major impact on the downhill course of most common malignancies. Residual tumor cells that bypass even radical combinations of surgery, chemotherapy, and radiotherapy can lead to relapse, with an even more aggressive behavior. Moreover, the issue of the multidrug-resistant phenotype that some cancer cells acquire is a major obstacle . This frustrating state-of the-art behooves us to double our efforts in the search of innovative treatment avenues.
During the past decade, cancer gene therapy has been one of the most exiting areas of therapeutic research, and some research has led to advanced stages of clinical trials with promising outcomes. The clearest advantage for this targeted therapy approach is that normal tissue toxicity may be avoided if suitable targeted delivery and tumor-specific expression strategies are employed. The issue of very efficient targeted delivery, which is ultimately needed for successful cancer therapy, still poses a major challenge, and enormous effort has been made to overcome this challenge. Major advancements have been achieved with regard to the tumor-specific expression strategy, also called transcriptional targeting, as a result of great efforts to identify genes that are expressed specifically in tumors and marginally in normal cells. Different strategies to improve and design tumor-specific promoters have been reported . In this approach, the accumulated understanding of gene regulation has been exploited to target the diseased cells successfully with the therapeutic gene expression. Other approaches for targeting tumor cells specifically have also been developed. The transductional targeting approach involves the genetic modification of viral vectors to enhance its tropism to achieve selective delivery of transgenes to target cells, and it may also result in cancer cell lyses when using oncolytic viruses. To achieve maximal specificity, transcriptional targeting may be combined with transductional targeting. Targeted therapy also involves small-molecule drugs and monoclonal antibodies. In the latter case, toxic molecules can be delivered specifically to tumor cells through monoclonal antibodies by exploiting our knowledge of the unique antigens that are expressed on tumor cells . Moreover, cancer gene therapy approaches can be administered in combination with chemotherapy and radiotherapy.
This chapter focuses on the successful use of H19 gene regulatory sequences for transcriptional targeting of cancer cells. The heterologous H19 promoter is transactivated by the same transcription factors and complexes that drive its transcriptional upregulation in the course of malignancy. The next step after determining high expression levels of H19 in numerous types of cancer was to characterize H19’s regulatory sequences. In our group, cloning of different fragments of the human H19 promoter was done and enabled the examination of their transcriptional activity in a variety of cancer cell lines. The region located between −229 and −134 bp contains a sequence element(s) with a positive transcriptional activity. The region between 85 and −61 bp is rich in CpGs, and it plays a significant role in the regulation of H19 transcription. This region contains transcription factors binding sites, including the CCATT box, which binds transcription factors from the C/EBP family. The sequence region between −85 and −134 bp acts as a binding site for negative acting transcription factor(s) in carcinoma cells .
Therefore, expression of the therapeutic toxin—in our case, diphtheria toxin A driven by H19 promoter—is predicted to occur in a manner specific for tumor expressing or overexpressing H19 gene product. This approach has been applied in clinical trials in patients of bladder, ovarian, colon, liver metastases and pancreatic carcinomas with promising outcomes . Preclinical validation of this approach is under development for other types of human carcinomas.
Therapies that target the H19 RNA or that exploit its transcriptional regulatory element to drive the expression of a cytotoxic gene specifically to tumor cells would therefore be very valuable in treating tumors that have a high level of H19 RNA. However, the potential of this transcriptional targeting approach might be even greater if two different regulatory sequences, selected from the cancer-specific promoters H19, IGF2-P3, and IGF2-P4, are utilized in the same therapeutic construct because many tumors that lack H19 gene expression have been found to express IGF2. In a second approach, a single promoter may be used to drive two cytotoxic genes having synergistic effect on a single construct. Both approaches have shown superior tumor growth inhibition activity in preclinical studies of bladder cancer .
H19 and Tumorigenicity
Understanding the function of the H19 gene is crucial if we hope to uncover its roles in cancer and harness this knowledge for therapeutic benefit. The H19 gene was the first imprinted noncoding RNA gene to be discovered . It is highly expressed in most fetal organs but repressed immediately after birth . Regarding its role in tumorigenicity, accumulating data from our group and others support the oncogenic function of H19 RNA, although several groups have reported H19 as having a tumor suppressor activity . The H19 locus harbors the potential to produce various products that may account for this discrepancy. From H19 exon 1, miR675 is produced . However, this microRNA is predicted to have a tumor-promoting role by targeting retinoblastoma tumor suppressor . Moreover, a short-lived, approximately 120-kb-long antisense transcript called 91H is produced at the imprinted H19/IGF2 locus, overlapping the H19 gene. 91H transcript is stabilized in breast cancer cells and overexpressed in human breast tumors . Recently, an antisense transcript encoded by H19 has been identified and called HOTS (H19 opposite tumor suppressor); it is a protein coding transcript and has a probable tumor suppressor function .
H19 RNA is emerging as one of the key players in cancer biology. Although H19 was long ago reported to show aberrant expression in various types of human cancer, where the majority of human tumors frequently express the H19 gene, clear, causal evidence of its role in cancer has only recently come to light.
H19 RNA contributes significantly to several aspects of the malignant phenotype, including proliferation, hypoxic stress response, angiogenesis, metastasis, and multidrug resistance . Cancer cells devoid of H19 expression encounter a very significant retardation of tumor growth in vivo .
To shed light on H19’s mechanism of action, which remained obscure until recently, we and others utilized a strategy of identifying upstream effectors and also downstream targets by applying the global gene expression profiling to identify genes modulated by both H19 overexpression and knockdown. Consequently, our group and others identified numerous modulators of H19 gene expression, including chemical carcinogens (e.g., BBN and DEN); retinoic acid; steroid and peptide hormones; growth factors such as hepatocyte growth factor; transcription factors such as E2F, HIF1α, p53, and c-myc; and environmental factors such as hypoxia and serum starvation. Because a complete report is beyond the scope of this concise review, for in-depth discussion, readers are referred to references . Here, we discuss principal findings.
The identification of molecular mechanisms used to mediate the hypoxic cancer cellular response is of great interest to identify targets that might compromise the survival of hypoxic cells. Hypoxia is a major trigger for tumor angiogenesis, invasiveness, metastasis, chemoresistance, radioresistance, and loss of genomic stability. It is also associated with poor prognosis in some types of human cancers. Notably, most of these conditions are associated with an increase in the level of H19 RNA.
Recently, we uncovered a molecular mechanism that integrates H19, p53, and HIF1-α to hypoxic stress response . We demonstrated a tight correlation between H19 RNA elevation by hypoxia and the status of the p53 tumor suppressor. p53 suppresses H19 elevation in hypoxic cancer cells. Furthermore, we identified HIF1-α as the factor responsible for H19 elevation under hypoxic stress in the tumor cells in which p53 is mutated .
Consequently, H19 functions as a stress modulator and a survival factor, and it is involved in several fundamental processes, including epithelial–mesenchymal transition (EMT), malignant transformation, cell cycle transition, metastasis, and neo-angiogenesis . EMT is an important process on the way to the malignant phenotype; notably, H19 upregulation occurs in the stroma as well as in the epithelium . In the metastatic tumor stage, which bears a striking similarity to the embryonic stage, H19 involvement appears to be essential: Adherent and cohesive cells lose their anchorage, migrate under stressful conditions to remote sites, and replicate with neovascular support. Thus, H19 seems to occupy a central role in the cancer embryonic shift .
Because hypoxia readily occurs in the majority of solid tumors driving critical steps in tumor development and metastasis and resistance to therapeutic modalities, placing the H19 gene product in this deadly circuit undoubtedly will have major impacts in its utility as a high-priority target for cancer gene therapy.
Targeting Toxins and Suicide Genes in Gene Therapy
By definition, a suicide gene has a product that will cause a cell to kill itself through apoptosis. This product can be transcribed by a variety of promoters (e.g., constitutive promoters and tissue-specific promoters), but tumor selectivity can be approached through the utility of tumor-specific promoters. The specificity of promoter-targeted therapy can also derive from cancer-specific conditions such as hypoxia . Inducible promoters affected, for example, by radiation, heat, and drugs have also been reported .
Finding a promoter that uniquely directs expression in cancer cells such as in the case of H19 is of great importance. Another example is the human telomerase reverse transcriptase (hTERT), the catalytic subunit of the telomerase—a critical factor for cell immortalization and tumorigenesis. As demonstrated by our group and others, the use of the hTERT promoter has provided targeted preclinical therapeutic results in bladder and hepatocellular carcinoma cells . Moreover, our group used promoters of IGF2 gene for this purpose .
The main safety advantage of specific promoters therapy is sometimes accompanied by the disadvantage of weak activity, consequently resulting in a decrease in therapeutic efficacy. To improve specific but weak promoters, enhancers can be added and negative regulatory elements can be removed. For example, by insertion of four tandem copies of the synthetic androgen responsive element, a nearly 20-fold enhancement of activity over the native PSA promoter and enhancer (PSE) was achieved . In the case of H19, this promoter has the great advantage of its specificity to cancerous cells and at the same time demonstrates strong promoter activity similar to SV40.
Targeted therapy also involves small-molecule drugs and monoclonal antibodies. Monoclonal antibodies that recognize, for example, the CD20 molecule (tositumomab and 131 I-tositumomab (Bexxar), and ibritumomab tiuxetan (Zevalin)) and CD30 molecules (brentuximab vedotin (Adcetris)) linked to toxic molecules have been approved by the U.S. Food and Drug Administration (FDA).
Toxin Gene Therapy
Toxins have the ability to kill cells efficiently; thus, many toxins have been examined as potential anticancer agents. Targeted fusion toxins consist of a targeted protein such as a growth factor fused to a bacterial toxin such as diphtheria toxin. The fused toxin is directed to the tumor cells via the targeting molecule, directed into the cells through receptor endocytosis, and then the toxin is released, resulting in tumor cell death.
Diphtheria Toxin A Chain
Diphtheria toxin (DT) is one of the most studied molecules, demonstrating compelling activity as a suicide gene therapeutic reagent. It efficiently ADP-ribosylates elongation factor-2 (EF-2) and thus blocks the translational machinery of target cells. It is estimated that a single molecule of diphtheria toxin can kill target cells, and many studies have successfully used its toxicity to eradicate target cancer cells. Diphtheria toxin is secreted from Corynebacterium diphtheriae as a single polypeptide chain containing two major domains: DT-A, which carries the active site for ADP ribosylation of EF-2, and DT-B, which promotes binding of toxin to cells and the entry of the A chain into the cytosolic compartment. Although a very low level of DT-A expression suffices for cell killing, DT-A released from the lysed cells is not able to enter the neighboring cells in the absence of the DT-B chain The advantages of DT-A in gene therapy are described in the literature and include (1) high potency, with one molecule able to kill a cell; (2) independence of cell cycle and p53 status; (3) toxic effect localized to transfected cells because the DT-B chain, which is responsible for cell penetration, is absent; (4) bypassing of anti-DT immunity because DT-A protein is being produced endogenously via an expression cassette within the tumor cells (to escape neutralization by anti-DT antibodies that are ubiquitous in most people during systemic administration); and (5) the absence of cellular resistance to the toxin. All of these characteristics make the DT-A chain a very effective and thus frequently used component of targeted cancer therapeutic approaches including immunotoxins (protein conjugates of DT-A combined with either an antibody or a cytokine to specifically target delivery to cancer cells) and in gene therapy studies ( Table 8.1 ).
| Full Name | Promoter/Immunotoxin/Protein | Target Tumor Type | Summary | References |
|---|---|---|---|---|
| BC-819 | H19 | Bladder, ovarian, pancreas, colon liver metastases | Drives the expression of the therapeutic protein, the DTA chain. It has been used in both animals and humans. | |
| hTERT-DTA | hTERT promoter | Bladder and hepatocellular carcinoma cells | Expression vectors containing the DTA gene were linked to hTER and hTERT transcriptional regulatory sequences, and inhibition of protein synthesis occurred in bladder and hepatocellular carcinoma cells. | |
| Denileukin diftitox (Ontak) | Interleukin-2 (IL-2) | Cutaneous T cell lymphoma | The drug binds to cell surface IL-2 receptors, which are found on certain immune cells and some cancer cells (expressing the CD25 component of the IL-2 receptor), directing the cytotoxic action of the diphtheria toxin to these cells. (FDA approved for use in humans). | |
| Rad51-DTA | RAD51 | The majority of human tumor cells, including those of the prostate, pancreas, breast, lung, and cervix | RAD51 is a recombinase protein essential in repairing DNA double-strand breaks and stalled replication forks by homologous recombination. Rad51-DTA/jetPEI injections treat tumors in mice with HeLa xenografts (subcutaneous and i.p. tumor). | |
| pMSLN/DTA, pHE-4/DTA | MSLN and HE4 promoters | Ovarian | The promoter sequences of two genes that are highly active in ovarian tumor cells, MSLN and HE4, were used to target DTA expression to tumor cells. Administration of DTA nanoparticles directly to s.c. xenograft tumors and to the peritoneal cavity of mice bearing primary and metastatic ovarian tumors resulted in a significant reduction in tumor mass and a prolonged life span compared to control mice. | |
| PGL3-DF3-DTA | DF3 promoter | Breast cancer | Recombinant expression vector containing human breast cancer DF3 promoter and diphtheria toxin A fragment was highly expressed in human breast cancer cell line of DF3 positive, and it could kill the human breast cancer cells not only in vitro but also in vivo . Thus, it could produce a specific killing effect on human breast cancer cell line of DF3 positive. | |
| EGF-DTA | EGF | Glioma cells | Recombinant toxin consisting of EGF fused to diphtheria toxin (DAB389EGF) effectively kills glioma cells, and this molecule is viewed as a promising agent for treating malignant gliomas–primary brain cancers. | |
| pAF-DTA | Human α-fetoprotein (AFP) promoter/enhancer | Hepatocellular carcinoma cells | DTA under the control of human AFP promoter/enhancer directed to AFP-producing hepatocellular carcinoma cells (using cationic liposomes (DMRIE-C)). After pAF-DTA transfection, the growth of AFP-positive HuH-7 cells was inhibited. Also, the growth of HuH-7 transplanted on BALB/c nu/nu mice was inhibited by i.t. injection. | |
| E-selectin-DTA | E-selectin promoter | Human endothelial cells (HUVEC) | HUVEC were killed via apoptosis. Maxwell et al . proposed that delivery of transcriptionally regulated expression plasmids for DTA in vivo , using cationic lipids that show preferential accumulation in activated or proliferating endothelium, may offer a novel means of inhibiting undesired angiogenesis. | |
| hCG-DTA | Human chorionic gonadotropin promoter | Malignant ovarian cell lines | DTA-chain gene regulated by the hCG promoter directed to malignant ovarian cell lines demonstrated the preferential expression of the DTA and provides an avenue for targeting such cells for suicide, toxin, or cytokine genes. | |
| Mesothelin-DTA | Mesothelin | Pancreatic cancer cell lines | Mesothelin is specifically overexpressed in pancreatic cancers and not in the adjacent normal tissue. Showalter et al . sought to target mesothelin-expressing pancreatic cancer cells with a potent suicide gene, DT-A. This work achieved dramatic inhibition of protein translation (>95%) in mesothelin-expressing pancreatic cancer cell lines. | |
| pPSA-DTA | Degradable, poly(β-amino ester) polymer, poly(butane diol diacrylate co-amino pentanol) (C32), DTA driven by a prostate-specific promoter | Benign prostatic hyperplasia (BPH) and prostate cancer | Using a degradable, poly(β-amino ester) polymer, C32, DTA driven by a prostate-specific promoter was delivered to cells and the same C32/DTA nanoparticles were directly injected to the normal prostate and to prostate tumors in mice. Nearly 50% of normal prostates showed a significant reduction in size, attributable to cellular apoptosis. These results suggest that local delivery of poly(β-amino ester) polymer/DTA nanoparticles may have application in the treatment of BPH and prostate cancer. |
Other Toxins
Plant-derived ricin and pseudomonas exotoxin use a similar mechanism as that of diphtheria toxin to kill target cells, and they have been examined as effective anticancer reagents .
As a selective pancreatic cancer suicide gene strategy, the use of suicide gene Escherichia coli purine nucleoside phosphorylase (ePNP) under the control of either CEA or MUC1 promoter sequences showed a preferential killing of CEA- and MUC1-producing pancreatic tumor cells .
In a comparative survival study, Rodriguez et al . showed the relative potencies of the following eight recombinant cellular toxins for irreparable prostate cancer cell death: Pseudomonas exotoxin A, ricin, tumor necrosis factor-α (TNF-α), diphtheria toxin (DT), Crotalus durissus terrificus toxin, Crotalus adamenteus toxin, Naja naja toxin, and Naja mocambique . Dose-dependent cytotoxic activity against all human prostate cancer cell lines tested was only identified as highly potent for ricin and DT. TNF-α had modest cytostatic activity in the screen; however, the combination of TNF-α and DT resulted in marked acceleration of the time to prostate cancer cell death . Our group demonstrated the cytotoxic effect of TNF-α cytokine, together with the diphtheria toxin, in the therapy of ovarian cancer. Intratumoral injection of the toxin vector into ectopically developed tumors caused 40% inhibition of tumor growth .
Suicide Gene Therapy Enzyme Prodrug Systems
Chemotherapeutic suicide gene therapy approaches are known as gene-directed enzyme prodrug therapy. Suicide gene therapy approaches using deactivated drugs are known as gene-directed enzyme prodrug therapy (GDEPT) or gene-prodrug activation therapy (GPAT).
GDEPT utilizes a gene encoding a foreign enzyme delivered to the tumor, after which a prodrug is administered and activates a cytotoxic drug that has been expressed in the tumor. Three of the most promising suicide gene/prodrug combinations are (1) herpes simplex virus thymidine kinase (HSV1-TK) with ganciclovir (GCV), (2) cytosine deaminase (CD) with 5-fluorocytodine (5-FC), and (3) bacterial nitroreductase (NTR) with 5-(azaridin-1-yl)-2,4-dinitrobenzamide (CB1954) .
Enzyme–prodrug systems kill the targeted cancer cells by interfering with the DNA replication or transcription processes. Moreover, the toxic substances produced by the combinations mentioned previously can spread to the neighboring cancer cells and induce consecutive cell death (the bystander effect). The two possible drawbacks of these enzyme–prodrug systems are that there is a prominent bystander effect and these systems tend to be less effective against cancer cells that are not actively dividing .
Another example of GDEPT is the CPG2–CMDA system. Kirn et al . developed a suicide gene therapy based on the bacterial enzyme carboxypeptidase G2 (CPG2). CPG2 has the advantage over the well-studied suicide genes HSV-TK and CD in that it activates prodrugs that are able to kill quiescent as well as proliferating cells. CPG2 cleaves the prodrug CMDA such that its cytotoxic drug is directly released and has the advantage that no further enzymatic processing is required for drug activation .
As mentioned previously, an important aspect of toxins/suicide gene therapy is the bystander effect, defined as the secondary effects on adjacent cells and tissues triggered by treatment of a primary target with a therapeutic agent. Such negative effect can be seen in this prodrug plasmid and virotherapy, which can affect the immune system. Thus, studies have been conducted using a polymer/biomaterial that mimics a virus but is safer as a delivery agent. However, it is important to note that whereas these plasmid prodrugs have significant bystander effect, DT-A—due to its properties as a toxin—is unable to re-enter a neighboring cell, has no bystander effect, and hence its role in gene therapy is significant.
Experimental Studies OF DTA-H19
The investigational agent BC-819, also referred to throughout this chapter as DTA-H19, is a double-stranded DNA plasmid of 4560 bp in length that carries the gene for the DT-A chain under the regulation of the H19 promoter sequence. This is a targeted cancer therapy because DT-A chain expression is triggered by the presence of transcription factors that upregulate H19 RNA expression in tumor cells. The selective initiation of toxin expression results in selective tumor cell destruction via inhibition of protein synthesis in the tumor cell, enabling highly targeted cancer treatment. DTA-H19 is being developed for the treatment of cancers that have upregulated levels of H19 expression. The first four indications under development are transitional cell carcinoma (TCC) of the bladder, pancreatic cancer, ovarian cancer, and hepatic cancer (either primary hepatic cell carcinoma or hepatic metastases of other primary cancers). DTA-H19 is administered intravesically into the bladder of TCC patients after mixing it just prior to intravesical instillation with the cationic transfection agent polyethyleneimine (PEI) ( in vivo jetPEI) to enhance in vivo transfection efficiency. For other indications cited previously, DTA-H19 is given by local/regional routes of delivery (intratumorally, intraperitoneally, or by hepatic artery infusion (HAI)), and in these cases it is not previously mixed with PEI but injected as naked DNA. In all clinical trials, patients’ tumors must be tested and be positive for H19 RNA in order to be eligible for treatment.
Nonclinical Pharmacology
Nonclinical studies ( in vitro/in vivo ) have been performed to demonstrate the potential efficacy of BC-819 in the different targeted cancers (bladder cancer, pancreatic cancer, ovarian cancer, and hepatic metastases) having upregulated levels of H19 expression.
In vitro pharmacology studies started by analyzing the expression of the H19 gene in patients’ tumor tissues and in human tumor cell lines. Then, the selective expression of a reporter gene (luciferase) and/or the DT-A chain controlled by the H19 gene promoter was assessed in vitro at different human cancer cell lines expressing H19. Finally, the efficacy of BC-819 in terms of protein synthesis inhibition was assessed in tumor cell lines of various origins.
Once a proof of concept of efficacy was obtained in vitro , in vivo nonclinical studies were conducted in several animal models with BC-819 administered as a naked DNA (for ovarian, hepatic metastases and pancreatic cancer) and in a complex suspension with PEI (for bladder cancer).
In Vitro Nonclinical Pharmacology
Bladder Cell Lines
The cell-specific activity of the DT-A chain driven by the H19 promoter region was demonstrated in human bladder carcinoma cells (T24P) and human fibroblast cells (IMR-90). There was dose-dependent inhibition of luciferase expression (the reporter gene) in T24P cells starting at 0.1 μg of co-transfected BC-819 and complete inhibition with 1.0 μg. This demonstrated that luciferase production was inhibited in cells by the expression of the DT-A chain under the regulation of the H19 promoter only in cells that express H19 RNA (i.e., T24P bladder cancer cells) and not in cells that do not express H19 RNA (i.e., the IMR-90 fibroblast cell line). It was further demonstrated that the inhibition of luciferase expression is indeed attributable to the toxic effects of the DT-A chain because this effect was not observed in 293-DT-A-resistant cells .
Pancreatic Carcinoma Cells
Two human pancreatic carcinoma cell lines expressing high levels of H19 (CRL 2547 and CRL 2119) were co transfected with 2 μg of LucSV40 and different concentrations of BC-819 vector. This experiment demonstrated that DT-A expression resulted in successful inhibition of protein synthesis as reflected by the decrease of luciferase activity in pancreatic tumor cells cell lines expressing H19 .
H19 Expression in Pancreatic Tumors
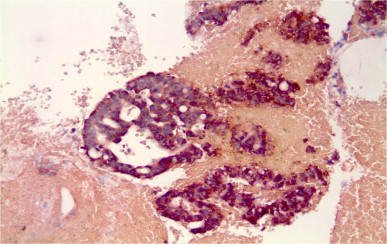
Pancreatic tumors from 36 patients and normal distal pancreatic tissue of 7 patients with tumors (obtained from the pathology department at Hadassah Medical Center, Israel) were examined for H19 expression by in situ hybridization (ISH) and/or polymerase chain reaction (PCR). Of the 36 tumors tested, 70% were positive for H19 expression.
According to the results obtained from the biopsies of the 16 patients with unresectable pancreatic cancer screened in the phase I/IIa study, 90% of the patients showed H19 expression in the tumor. A representative example of ISH of a pancreatic tumor is shown in Figure 8.1 . No H19 expression was found in normal pancreatic tissue distal to tumor regions .