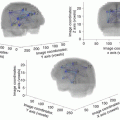
Fig. 5.1
Three examples (a = case 1; b = case 2; c = case 3) of microfoci of “intermediate grade” at low magnification (left) and at high magnification (right) with the low grade glioma in background
In a previous study, we investigated whether these foci displayed histological or immunohistochemical (IHC) similarities to GIII, such as hypoxia, high proliferative activity, increased vascularization, and activation of oncogenic pathways. For this purpose, we compared the histological, immunohistochemical and molecular features of these heterogeneous gliomas (called “GII+” in this study) to those of homogeneous GII and anaplastic GIII [3].
It is now well established that the grade II gliomas are premalignant tumors that will ineluctably evolve towards higher grade of malignancy.
The current WHO classification [1] however describes GII and GIII tumors as clearly distinct entities. This clear-cut distinction overlooks the possibility of a continuum between GII and GIII tumors. Moreover, the morphological border between GII and GIII is not precisely defined in the WHO classification. For instance, the notions of “prominent microvascularisation” or “high cellularity” are subjective and do not give precise parameters of evaluation.
We find the main limitation of the WHO grading of diffuse gliomas to be the lack of an intermediate grade between grade II and grade III as we often see tumors that in fact exhibit features in between the two grades. It seems logical and obvious that there is a biological continuum between the grades, and we purpose the use of an intermediate term in the future. The presence of the described foci of increased cellularity and proliferation of vessels may correspond to grade II in transformation to grade III. It is interesting to note that while some show signs of cellular atypia, others show signs of neoangiogenesis [3].
5.3 Discussion
As mentioned, because the cognitive performance of the patient is under control during the procedure, awake neurosurgery allows extensive resection of gliomas. Large and non-damaged samples are therefore available for histopathological evaluation. We noticed the recurrent presence of hyper cellular micro foci in GII specimens obtained by awake neurosurgery. We theorized that those foci might be the early signs of malignant progression to GIII. Aggressiveness in gliomas is probably underestimated in biopsy specimen because of the intra-tumor heterogeneity. Indeed, some GII might contain transformation nests that are not visible by routine imaging techniques but that could be detected by pathological and IHC examination after surgery.
Hyper cellularity was a consistent feature of the foci, suggesting it might be the earliest anaplastic event, contrary as mentioned in the 2016 classification which describe a predictable sequence: atypia, mitosis, increased cellularity, and micro vascular proliferation and/or necrosis. The results of our IHC and molecular investigations were in favor of the hypothesis of a transition from GII to GIII initiated by the generation of small hypercellular or hypervascularized areas. Indeed, in all the GII+ cases studied, we observed several alterations that were present only in the foci and not in the tumor tissue background. We observed that the proliferation index and the vascularization of GII+ foci -measured objectively using digital calculation- were close to those of GIII whereas they stood out from those of the GII background. In addition, in two cases, we observed cells expressing HIF-1α in the foci but not in the background in two cases of GII+ tumors [3]. It revealed hypoxic conditions specific present in the foci and might be a sign of pejorative evolution since HIF-1α expression has been correlated to higher grade and poor prognosis in astrocytic tumors [4]. In our experience, the increased vascularization and hypoxia were not correlated clinically to the occurrence of MRI enhancement [3]. This might be explained by insufficient sensitivity of MRI in regard to the very small size of these foci. Contrast enhancement may only detect late events whereas histological examination allows the detection of this early subclinical change reflecting neoplastic progression. To note, specific techniques of spectroscopy or perfusion MRI [5, 6] and techniques of nuclear medicine, such as dynamic 18F-fluorethyltyrosine (18FET) positron emission tomography (PET), could also be promising to identify high risk in low-grade gliomas [7].
Moreover, other authors [8] showed that dynamic contrast-enhanced MRI was helpful to predict the presence of areas of increased hypoxia and proliferation in gliomas. Therefore, we assume that microscopic evaluation of the vascularization associated with cell proliferation in GII+ micro foci, combined with nuclear imaging, might become central in managing the care of GII patients.
We have demonstrated that the foci of GII+ cases displayed increased proliferation and cellularity, vascular hyperplasia and signs of hypoxia. In addition, we identified oncogenic alterations in the foci [3]. Alterations of EGFR-activated pathways are known to be involved in gliomas tumor genesis. Amplification of EGFR has been described in 45% of glioblastomas and in 10% of GIII whereas it is not reported in GII [9, 10]. EGFR overexpression is commonly found in low-grade astrocytomas and oligodendrogliomas [9]. While we did not observe amplification of EGFR, we observed overexpression of EGFR. It has been reported that EGFR expression increases with the grade of malignancy [11, 12]. Overexpression in the absence of amplification can be explained by point mutations or by non-genomic deregulation, such as transcriptional, translational or post-translational changes. However, it might also be caused by differences in the sensitivity or specificity of IHC and FISH methods [13]. AKT is a downstream effector of the tyrosine kinase receptors EGFR and PDGFRA. Its activation plays a role in glioma progression and is the most frequent alteration in glioblastomas (cancer genome atlas). The expression of P-AKT has been shown to correlate to the grade of the gliomas [14]. It has also been correlated to angiogenesis [15]. We detected an expression of EGFR and P-AKT significantly higher in the foci than in the background in eight and five cases of GII+, respectively. All the five foci that showed a positive expression of P-AKT displayed an overexpression of CD31, a marker of angiogenesis, while four of them overexpressed EGFR (Hirsch’s score > 200) [3]. This suggests a synergy of these different alterations. Altogether, the foci showed activation of the EGFR-PI3K pathway at various levels. The activating mechanisms other than EGFR amplification, such as inactivation of PTEN, activating mutation PI3KCA or amplification of AKT need to be further investigated in order to understand the causes of EGFR pathway activation [16, 17].
TP53 mutations have been described as major events in the process of tumor initiation. They are predominantly observed in secondary glioblastomas [18]. They are considered as an early alteration in glioma genesis. The intensity of anti-P53 immunostaining, which detects abnormal forms of the protein accumulated in the nucleus, has been correlated to the astrocytoma type and to tumor grade [19]. We have shown the intensity of the P53 staining in the foci to be higher than in the background in two GII+ cases [3]. This suggests that clonal mutations of TP53 might have arisen in the foci. TP53 positive tumor cells of the foci could therefore harbor genetic instability leading to anaplasia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree