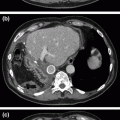
Fig. 32.1
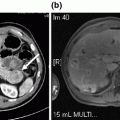
Diffuse small duct pancreatitis is demonstrated in this CT scan of a patient with idiopathic chronic pancreatitis who underwent total pancreatectomy with islet autotransplantation. Noted are a prominent, mildly dilated main pancreatic duct, parenchymal fibrosis, and punctate calcifications
Diabetes
Patients with chronic pancreatitis may develop pancreatic endocrine failure due to islet cell loss (type 3c diabetes). Patients with diabetes may still benefit from islet autotransplantation, as endogenous c-peptide production can ameliorate the severity of resultant surgical diabetes after pancreatectomy [7]. Patients with diabetes on evaluation for TPIAT should be evaluated for their level of islet function, however, to help weigh whether the potential benefits of islet autotransplantation warrant the risks inherent. Stimulated c-peptide response should be assessed, often with a mixed-meal tolerance test.
Nutritional Assessment
Patients with chronic pancreatitis are at risk for nutritional failure due to poor PO intake and from pancreatic exocrine insufficiency. A full nutritional evaluation and efforts to optimize nutritional status preoperatively are important for best outcomes. Pancreatic enzyme replacement therapy is best instituted preoperatively. Evaluation for malnutrition with assessment of serum albumin and pre-albumin levels are requisite. Preoperative oral supplementation or enteral feeds may be required in the severely affected. Deficiencies in the fat-soluble vitamins should be addressed.
Physiologic Assessment
As with other major elective abdominal operations, attendant comorbidities, including cardiac, pulmonary, and renal disease should be evaluated when considering patient candidacy for surgery. In particular, a history of hepatic disease is important, as the islet infusion is an embolic event, which may be a significant stressor to a previously compromised liver, increasing morbidity.
Behavioral Medicine Evaluation
Patients evaluated for TPIAT should undergo comprehensive psychological assessment to evaluate relative preparedness for surgery [8]. Evaluation assesses multiple psychological domains, including global cognitive functioning, quality of life, and coping. The patient’s knowledge and expectations of the procedure are assessed, as well as the support system available. A complete psychiatric evaluation is performed to diagnose previously untreated psychiatric disorders, e.g. depression, anxiety. The patient’s health behaviors and compliance are also assessed to screen for narcotic dependence/abuse and ability to manage complex medication regimens, e.g., pancreatic enzyme therapy, insulin.
Preoperative Counseling
In consideration of this radical, elective procedure, patient education is paramount. Patients should demonstrate understanding of proper expectations. The goals of the procedure are pain relief and improvements in quality of life. These expected outcomes come at the cost of lifelong (ameliorated) surgical diabetes and exocrine insufficiency.
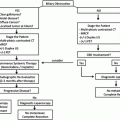
Patient Selection Criteria for Total Pancreatectomy with Islet Autotransplantation for Chronic Pancreatitis
1. Chronic pancreatitis
Evidenced by: CT, MRCP, EUS (5 criteria, high probability), or prior surgical pathology
OR
Recurrent acute pancreatitis
Evidenced by: at least two documented episodes with lipase >3 time normal
2. Debilitating pain
Defined by daily narcotic use and/or inability to work, attend school, or engage in normal societal roles
3. Not amenable to lesser interventions
Includes medical, endoscopic, and lesser surgical options
4. Physiologically fit
No prohibitive cardiopulmonary conditions, no significant hepatic disease
5. Psychologically fit
Requires behavioral medicine evaluation
Surgical Technique
The patient is positioned supine under general anesthesia. A triple lumen central venous catheter is placed for durable intravenous access, primarily in anticipation of the continuous infusions required during the initial postoperative period. Additional large-bore peripheral intravenous access is also established because there is the potential for rapid fluid shifts. An arterial line is placed and continuous hemodynamic monitoring is used to allow for goal-directed fluid management.
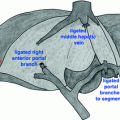
An upper midline laparotomy incision extending from the xiphisternum to above the umbilicus is typically used. A wound protector is utilized. The gastrocolic ligament is divided and the lesser sac is entered. The greater omentum and short gastric vessels are taken down along the greater curvature of the stomach. The hepatic flexure is mobilized and retracted inferomedially. A full Kocher maneuver is performed, mobilizing the duodenum to the level of the aorta medially. The right gastroepiploic vein and middle colic vein are identified and followed toward the superior mesenteric vein. The right gastroepiploic vein is divided, taking care to preserve the middle colic vein. The inferior margin of pancreatic neck is identified over the superior mesenteric vein, and the plane between the portal vein and the pancreatic neck is developed. The gastroduodenal artery is ligated, confirming continued perfusion of the hepatic artery. If the gallbladder is present, it is removed.
The common bile duct is dissected circumferentially and is divided. The stomach is divided proximal to the pylorus with a surgical stapler. Just distal to the ligament of Treitz, the proximal jejunum is divided and the jejunal mesentery as well as the ligament of Treitz are taken down with an energy device. The proximal jejunum and the fourth portion of the duodenum are then rotated under the superior mesenteric vessels.
The pancreatic neck is divided over the portal vein, and the pancreatic head is rotated laterally and dissected from the portal vein. The periarterial vascular and lymphatic tissue between the uncinate process and the superior mesenteric artery is divided, and the head of pancreas and duodenum are removed from the abdomen and immediately placed in cold balanced-electrolyte preservative solution.
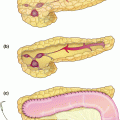
On the back table, the pancreatic head specimen is immediately prepared by flushing the gastroduodenal artery stump with cold preservative solution. The duodenum is removed and the pancreatic duct is cannulated with a 5 French pediatric feeding tube (Fig. 32.2a).
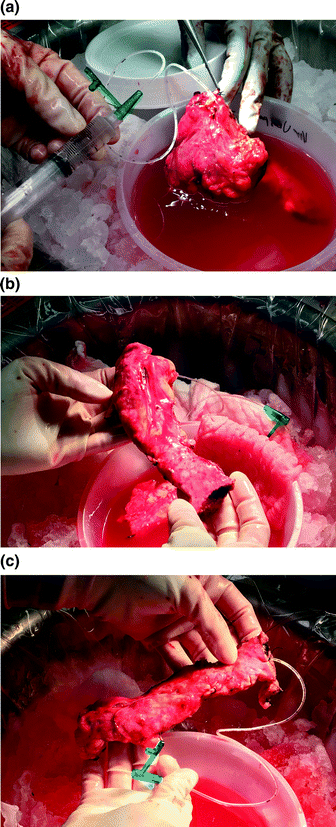

Fig. 32.2
a The head of the pancreas is prepared on the back table in cold preservation solution immediately upon extraction. The gastroduodenal artery is flushed to exsanguinate the organ. b Similarly, the body and tail of the pancreas are prepared. The splenic vein is opened and the splenic artery is flushed. c The main pancreatic duct of the prepared left pancreas is cannulated with a 5 French pediatric feeding tube, to be used in the cell processing lab for infusion of digestive protease
Next, attention is turned to removing the body and tail of the pancreas. The splenic artery is dissected and tagged but not yet ligated near its origin, the superior margin of the pancreatic body. Similarly, the splenic vein is tagged, taking care to preserve the coronary vein. Once the pancreas is dissected free from the surrounding structures, the splenic vessels are ligated and the distal pancreas and spleen are removed from the abdomen and placed in cold preservative solution.
On the back table, the spleen is removed sharply. The splenic vein is opened along its length to exsanguinate the specimen, and the splenic artery is flushed with cold preservation solution. The pancreatic duct is cannulated with a 5 French pediatric feeding tube, which is sutured in place (Fig. 32.2b, c). The pancreatic body and tail are packaged with the head, and the pancreas is then taken on ice to the islet cell laboratory for processing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree