Highlights
- •
Standard transperitoneal procedure is associated with high complication rates.
- •
Indications: elderly, frail, high risk patients.
- •
Second primary in the bladder with solitary functioning kidney.
- •
Complete avoidance of general anesthesia, minimal cardiopulmonary complications.
- •
Retrograde dissection posteriorly: caudal to cranial in the rectovesical space.
- •
End cutaneous ureterostomy for drainage of urine.
Abstract
Objectives
Radical cystectomy with urinary diversion is the gold standard treatment for bladder cancer (high-risk/muscle invasive). The transperitoneal approach is associated with significant gastrointestinal complications like ileus. In the elderly and frail with a single functional kidney, we describe an extraperitoneal technique of radical cystectomy, with a ureterostomy, to be performed without general anesthesia.
Materials and methods
The elderly, frail, and high-risk candidates for general anesthesia, with a prior history of nephroureterectomy with a second primary muscle-invasive bladder cancer, were chosen. All patients underwent the described procedure under combined spinal and epidural anesthesia. The posterior dissection was retrograde, caudal to cranial, with the peritoneum being opened only for resection of the dome. A cutaneous ureterostomy was fashioned on the side of the functional kidney. Peri-operative parameters were assessed for early recovery in this high-risk group.
Results
The mean age was 82 years (range: 73–91), with Charleson Comorbidity Index 5, and were all deemed unfit for neoadjuvant chemotherapy. With a median duration of 127.5 minutes, an average blood loss of 225ml, and no patient requiring general anesthesia; early ambulation, early return of bowel function, and a lesser hospital stay (7 days) with minimal morbidity were achieved. Negative surgical margins were achieved in all cases, with a mean harvest of 29 lymph nodes. Only 1 patient developed stomal stenosis. The cause-specific survival (CSS) is 100% at 2 years.
Conclusions
The highlighting features are the early return of bowel function (flatus passage on day 1) and the avoidance of the cardio-pulmonary complications of general anesthesia. The extraperitoneal cystectomy offers a promising alternative in this select group and warrants further studies to extrapolate this technique for bilateral urinary drainage.
1
Introduction
Bladder cancer is one of the most prevalent urologic malignancies in men, exhibiting a notably high incidence among elderly patients [ ]. For decades, radical cystectomy (RC) with urinary diversion has remained the gold standard treatment for non-metastatic muscle-invasive and high-risk non-muscle-invasive bladder cancer [ ]. This procedure offers excellent local cancer control, with low pelvic recurrence rates and 5-year cancer-specific survival rates ranging from 50% to 70% [ ]. Presently, the transperitoneal approach is the most widely adopted method, involving ante-grade bladder mobilization through the peritoneum via blunt dissection [ ]. Despite its popularity, this approach is plagued by high complication rates, ranging from 40% to 44% [ ]. The most prevalent complication categories include gastrointestinal (29%), infectious (25%), and wound-related (15%) complications, with ileus emerging as the predominant gastrointestinal complication, occurring at rates ranging from 17.8% to 23% [ ]. This elevated risk is attributed to increased exposure of the intestines to the atmosphere, tactile manipulation of bowel loops, and upward packing of the bowel loops for clearing the operative field during surgery. Preserving peritoneal continuity has emerged as a critical strategy in mitigating these postoperative complications [ ]. In 1999, Kulkarni et al. [ ] introduced a retrograde extra-peritoneal technique involving extra-peritonealization of the ileal bladder, aiming to reduce morbidity by separating extra- and intraperitoneal healing processes. Re-adaptation of the peritoneal layer resulted in earlier recovery of bowel function and fewer postoperative complications [ ].
In this manuscript, we present our experience with the open extra-peritoneal retrograde cystectomy, performed under combined spinal and epidural anesthesia, for patients who were at high risk for general anesthesia. A point to note in this study set is that all patients had a single, functional kidney. All the patients underwent a cutaneous ureterostomy on the side of the functioning kidney at the end of the procedure. This approach has lower operative time, lower rates of postoperative paralytic ileus, and minimal blood loss. This study focuses on the patient selection, the surgical technique, and the postoperative outcomes of our technique of retrograde extra-peritoneal cystectomy.
2
Patient selection
Candidates for this approach were carefully chosen based on a combined decision by the operating surgeon and the anesthesia team.
Inclusion criteria:
- •
All patients were confirmed to be non-metastatic muscle-invasive transitional cell carcinoma of the urinary bladder.
- •
The primary criteria included candidates who were less suited to general anesthesia in view of age, frailty, and cardio-pulmonary risk factors.
- •
The second specific inclusion was to limit patients with a single, functioning kidney (status post nephrectomy or nephroureterectomy for upper tract tumor), as it allowed for a single cutaneous ureterostomy, obviating the need to open the peritoneum to perform an ileal conduit or a neobladder.
Exclusion criteria:
- •
Candidates with prior lower abdominal surgery, anterior abdominal wall incisional hernia repair with mesh placement.
- •
Candidates amenable for partial cystectomy.
- •
Candidates who were deemed fit to undergo the standard procedure as per anesthesia.
- •
Candidates who had bilateral functional kidneys (which would require a bilateral drainage procedure – either a bilateral ureterostomy or an ileal conduit).
3
Methodology
All patients were evaluated as per the National Comprehensive Cancer Network (NCCN) guidelines [ ] for muscle-invasive bladder cancer. Preoperative imaging included Contrast-enhanced Computed Tomography of the abdomen and pelvis (CECT-A/P), High-resolution Computed Tomography (HRCT) of the thorax, and a transurethral resection biopsy to confirm tissue diagnosis. All patients were evaluated as appropriate for comorbid illness, specialist opinions were taken, and underwent a pre-anesthetic risk stratification based on the American Society of Anesthesiologists Physical status classification (ASA) [ ]. The Charleson Comorbidity Index (CCI) [ ] assessed the patient’s frailty.
A combined decision was taken by the operating surgeon, anesthesia team, and the patient before the planned procedure.
All patients underwent the surgical procedure on similar lines with minor case based variations as per intra operative findings.
3.1
Surgical technique
Position and Prerequisites: After induction of combined spinal-epidural anesthesia, the patient was positioned supine/ in a modified Lloyd Davies position and secured to the table at chest level. Vaginal packing was performed in female patients, and a 14Fr Foley catheter was inserted in a sterile manner. A moderate head low position displaces the bowel loops away from the pelvis.
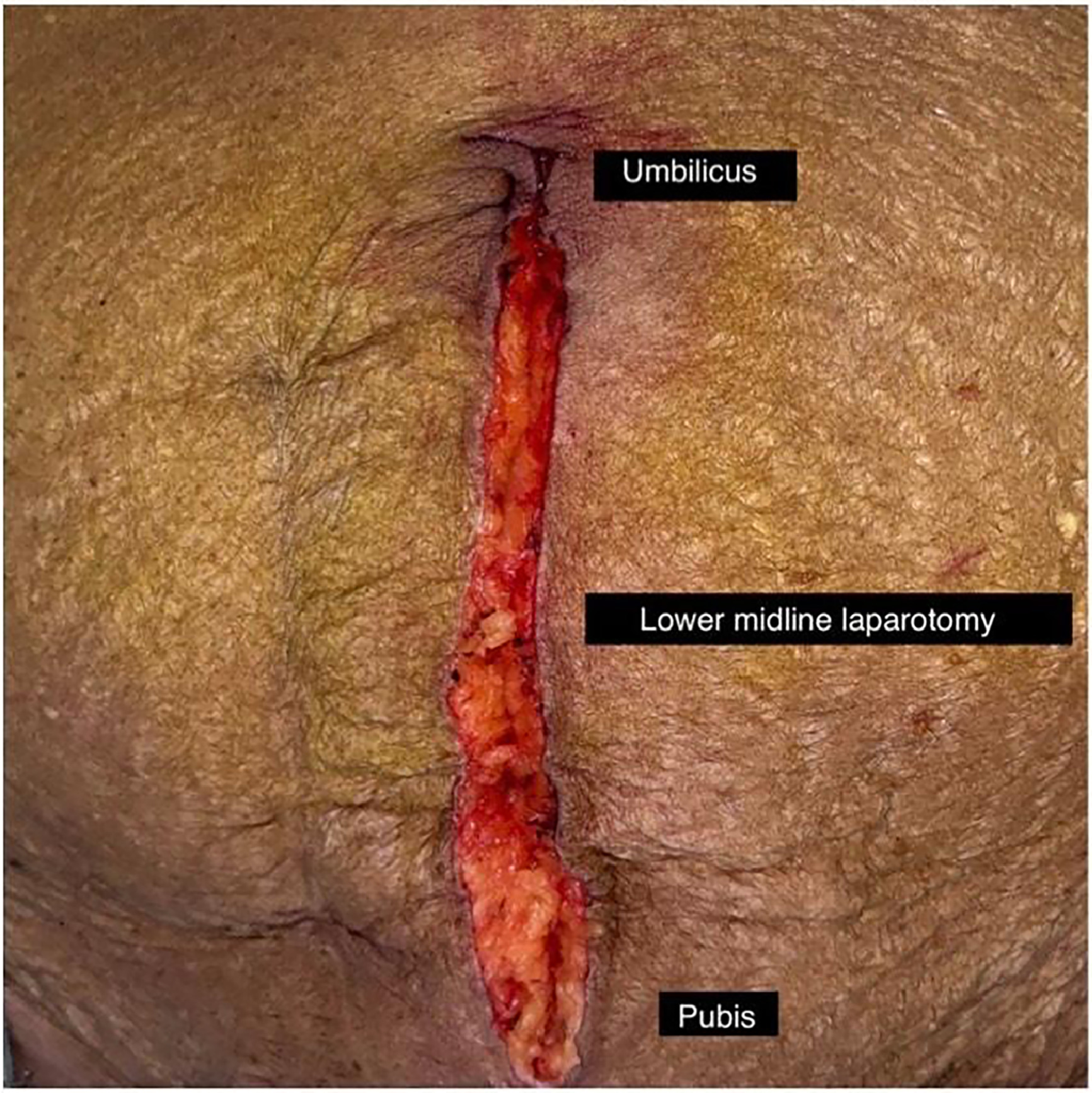
Incision: A lower midline incision was preferred ( Fig. 1 ), with the Pfannensteil incision being an alternative option. Following the skin opening and subcutaneous fat, the linea alba was incised to access the pre-peritoneal space.
Creation of Pre-Peritoneal Space: The pre-peritoneal space was cautiously dissected to avoid entry into the peritoneal cavity. Blunt and sharp dissection was employed to develop a plane extending cranially from the umbilicus, caudally up to the entry into the space of Retzius, and laterally up to the lateral surface of the external iliac vessels ( Figs. 2 A and 2 B).
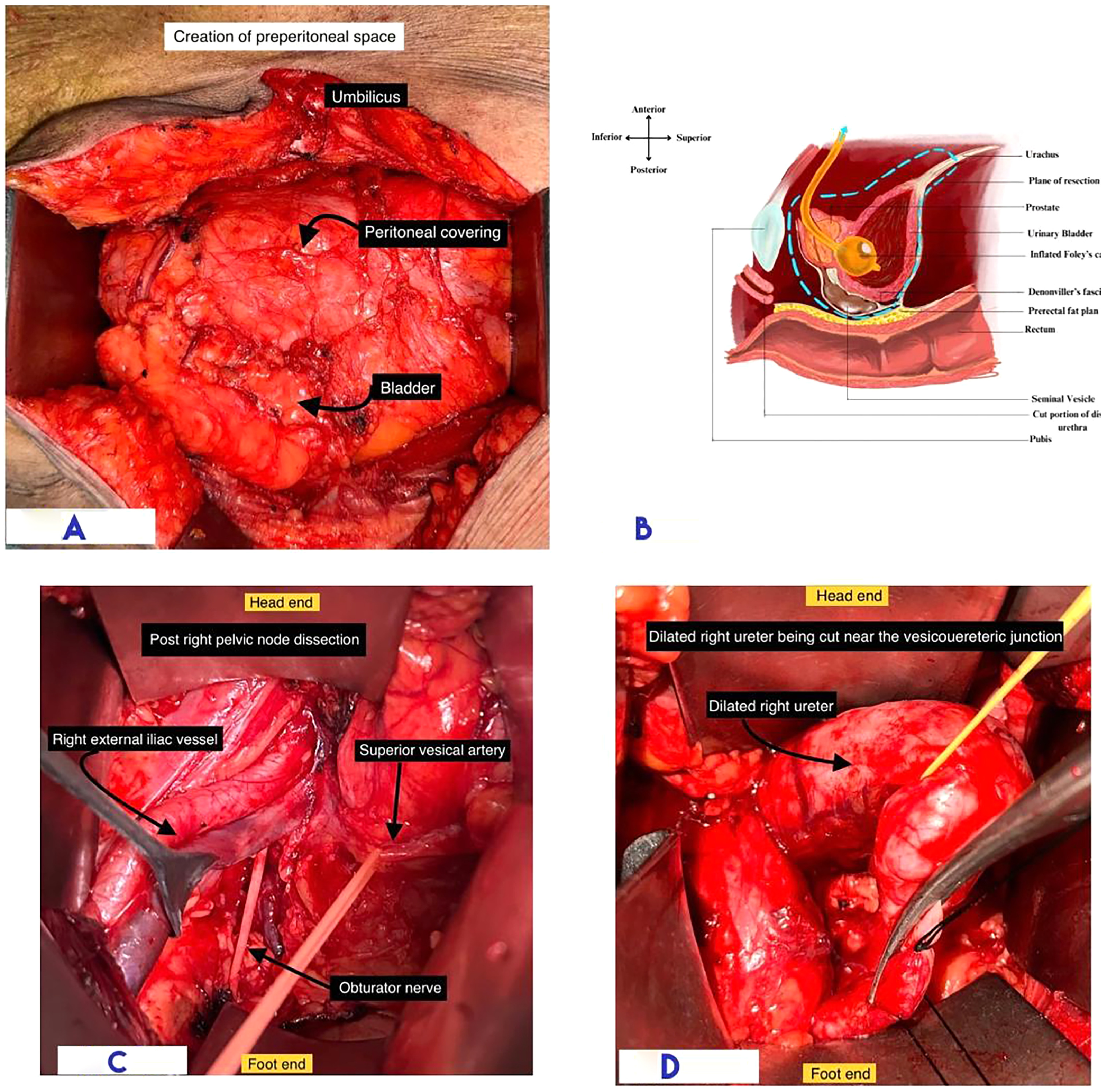
Pelvic Lymph Node Dissection: A bilateral standard pelvic lymph node dissection was performed ( Fig. 2 C). Performing lymphadenectomy early aids in better visualization and easier access for lateral and posterior dissection during the retrograde cystectomy.
Division of Superior Vesical Artery: The division of the superior vesical artery was the next step before tracing the ureter to facilitate dissection.
Dissection and Division of Ureter: Ureteric dissection proceeds from the crossing of iliac vessels to the junction with the bladder, ensuring preservation of its vascularity. The ureter was divided 1 cm proximal to the vesicoureteral junction ( Fig. 2 D), and the cut margin was sent for frozen section analysis.
Anterior and Lateral Wall of Bladder Dissection: Bladder mobilization was continued on both sides and anteriorly ( Fig. 3 A). The vas deferens on either side are cut, short of their entry into the prostatic urethra.
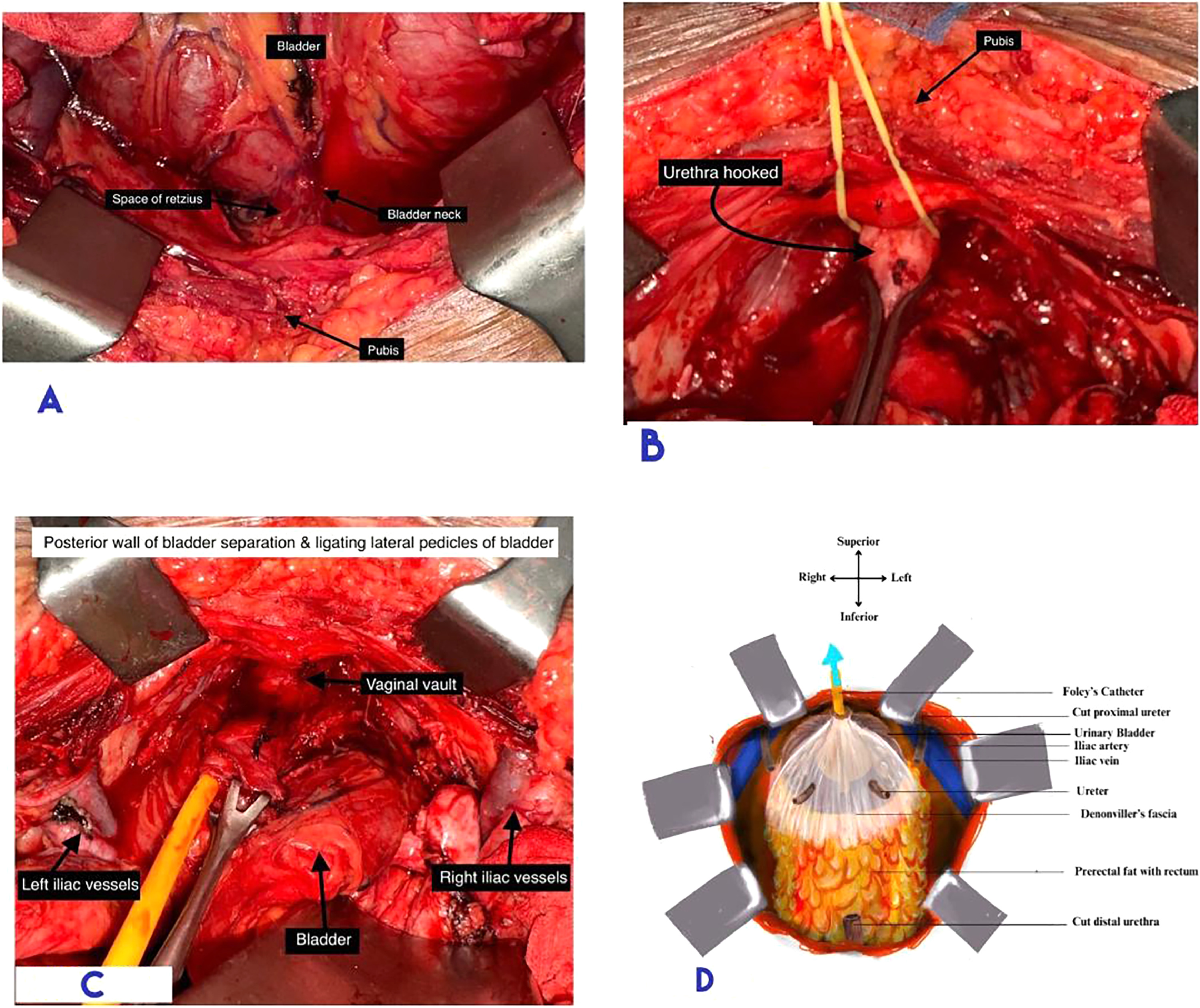
Division of Endopelvic Fascia: The endopelvic fascia was incised anteriorly to reach the prostatic apex in males and the bladder neck in females. The dorsal venous complex was suture ligated at this step.
Transection of Urethra: The anterior circumference of the urethra was transected. The Foley catheter was cut, leaving behind the bulb and a stump of tubing, which was used for traction during posterior dissection. The posterior circumference was cut once the Foley stump was secured for traction. ( Fig. 3 B)
The Retrograde Cystectomy: Retrograde cystectomy commences with urethral division, followed by separation of the bladder from the rectum with the Denonvillier’s fascia (in males) on the specimen side of the dissecting plane ( Figs. 3 C and 3 D). In females, a similar plane was developed anterior to the uterus, preserving the female organs of reproduction.
Lateral pedicles of the bladder were taken down using an energy device in a retrograde manner, caudal to cranial, reaching up to the peritonealzed surface. The bladder specimen was left hanging from the peritoneal attachment at the dome. ( Fig. 4 A)
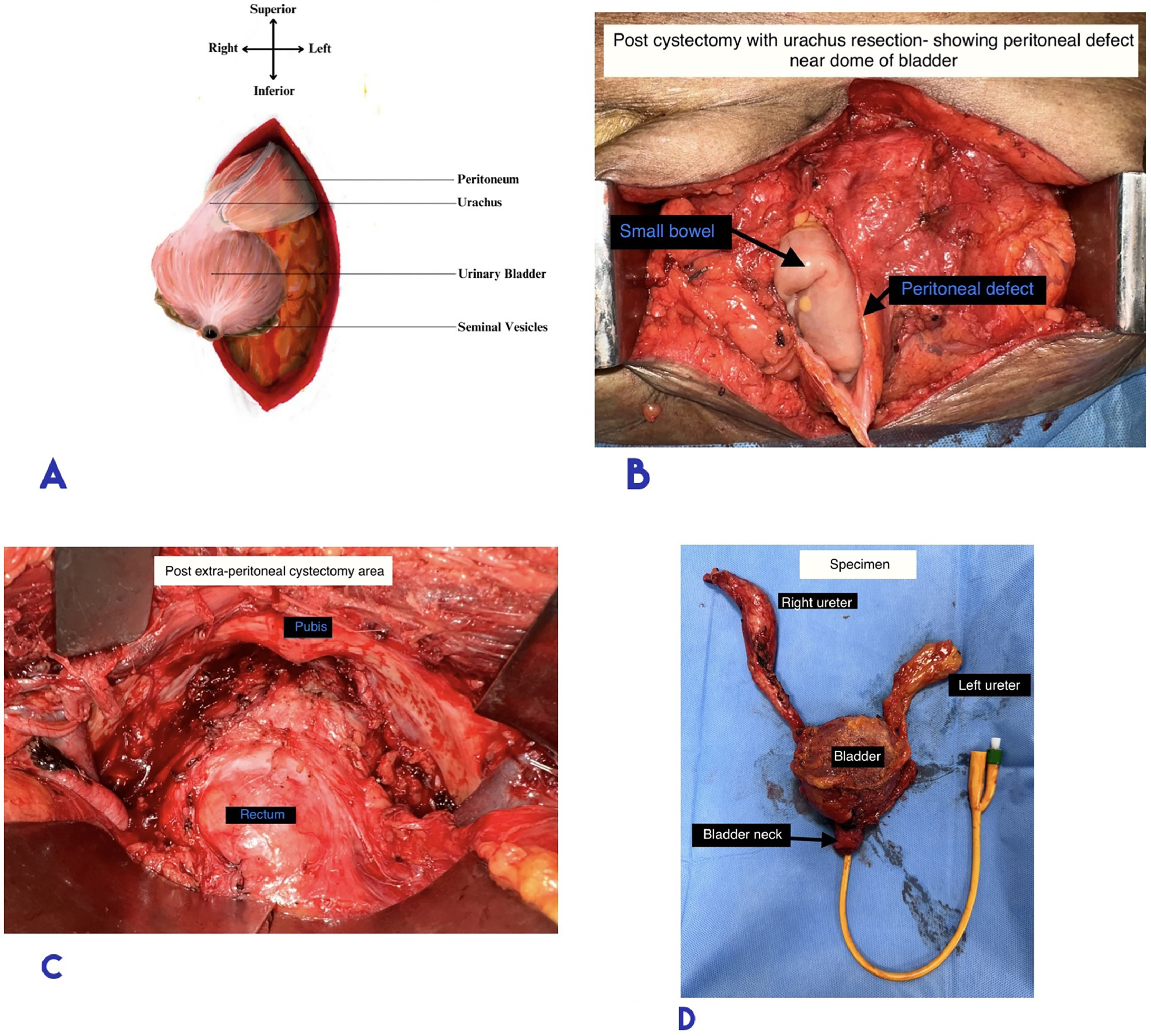
Removal of Bladder Along with Peritoneum over the Dome: Cystectomy was completed, leaving a peritoneal defect at the bladder dome, which was closed with Vicryl sutures ( Fig 4 B and 4 C).
The resected specimen ( Fig. 4 D) was comparable to cystectomy specimens obtained through the transperitoneal approach, with adequate soft tissue margins, intact distal ureter and bladder neck.
Cutaneous ureterostomy: The lumen was spatulated adequately and the ureterostomy was matured. A 6Fr double J stent was placed through the stoma, reaching up to the renal pelvis.
An abdominal drain was placed and the abdomen was closed in layers.
Intraoperative course: Vital signs were continuously monitored throughout the procedure. A major intra operative event was defined as at least one of the following features – tachycardia (heart rate >100 beats/min), bradycardia (heart rate <60 beats/min), any rhythm abnormality, hypotension, cardiovascular event requiring resuscitation, altered mentation, or conversion to general anesthesia.
Post-operative care: Patients were mobilized on post-operative day 1, initiated on oral liquids as tolerated on day 1, and advanced gradually. The drain was removed once the output was less than 100ml. The ureteric stent was retained for 12 weeks post-operatively until healing of the mucocutaneous junction.
The post-operative renal function was monitored with serum creatinine, serum electrolytes and urine output. Altered renal function was defined as per RIFLE criteria [ ]. The nephrologist’s opinion was taken as per indication and corrective measures were undertaken. Wound healing was assessed as per the Southampton Wound scoring system [ ], and the 30-day morbidity indicator was based on the Clavian Dindo score [ ].
Each patient was reviewed every 3 months. Clinical assessment, with chest X-ray, ultrasonography of the abdomen and urine cytology, was done to assess recurrences. An annual screening CECT-A/P with HRCT of the thorax was done, if renal parameters were acceptable. Noncontrast magnetic resonance imaging (MRI) was used for surveillance in patients with compromised renal function.
4
Results
Baseline characteristics of the 8 patients are summarized in Table 1 . All 8 patients were operated between January 2020 and June 2022. The majority were males (87.5%). Most patients were over 80 years (62.5%) with a median age of 82 years (range: 73–91). All patients were diagnosed with muscle-invasive bladder cancer with perivesical extension noted in 25% of patients on CECT A/P. All the patients were deemed unfit for chemotherapy given advanced age, comorbid illness, poor renal function (single kidney status), or performance status (Charleson Comorbid Index >5). After a pre-anesthetic assessment, 5 patients had an ASA score of 2, and 3 patients had a score of 3. { Table 1 }
| Factors | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Consolidation |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 73 | 75 | 84 | 91 | 77 | 88 | 83 | 87 | Mean–83.5 |
| Sex | M | M | M | M | M | F | M | M | M:F=7:1 |
| Charleson Comorbidity Index | Severe | Severe | Severe | Severe | Severe | Severe | Severe | Severe | Severe–8 |
| ASA score | 3 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | ASA2–5 ASA3–3 |
| Pre-operative clinical T stage | T3 | T3 | T3 | T2 | T3 | T2 | T3 | T3 | – |
| Neoadjuvant therapy (Y/N) | N | N | N | N | N | N | N | N | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree