(1)
Institut Bergonié, Bordeaux, France
Abstract
Toll-like receptors (TLR) constitute a family of receptors involved in immune and inflammatory processes, which recognise molecules derived from infectious agents (bacteria, viruses) and endogenous substances. They are studied in this chapter together with interleukin 1 (IL1) and its receptors (IL1R) and with related interleukins such as IL18 and IL33, with which they share a general activation mechanism. The activation of these receptors leads, after several steps involving cytoplasmic kinases, to the activation of transcription factors of the NFκB (nuclear factor κB) family, which activate the inflammatory response. NFκB can be activated in response to other signalling pathways, especially AKT in the PI3 kinase pathway (Chap. 3) or tumour necrosis factor (TNF). The signals generated by TLR/IL1R activation induce cell survival and proliferation: this is why the corresponding pathways can be diverted by oncogenesis processes.
The immune system contains two types of receptors able to recognise bacterial and viral components, called pattern–recognition receptors (PRR): membrane toll-like receptors and intracellular receptors called NLR (NOD–like receptors, NOD meaning nucleotide binding and oligomerisation domain). Activation of NLR activates in turn IL1 family interleukins, which, once secreted, induce an inflammatory response when recognised by cells equipped with their cognate receptors.
12.1 IL1 Family Interleukins and Their Receptors
The interleukins of this family are distinct from those studied in Chap. 4, because they are structurally different and induce a different signalling pathway. The IL1 family comprises 11 members distributed in four groups. In the IL1 group are IL1α (IL1F1) and IL1β (IL1F2) (genes IL1A and IL1B) as well as IL1RA (IL1 receptor antagonist) or IL1F3 (gene IL1RN), which cannot activate the signalling pathway and is therefore an inhibitor of this pathway. IL18 (IL1F4) and IL33 (IL1F11) each constitute a group; the IL36 group, recently identified, comprises IL36α (IL1F6), IL36β (IL1F8), IL36γ (IL1F9), IL37 (IL1F7), IL38 (IL1F10) and IL36RN (IL1F5), which is an antagonistic ligand as IL1RA. IL1 family interleukins are synthesised as precursors which are activated by proteolytic cleavage, by calpain for IL1α and by caspase 1 or ICE (IL1-converting enzyme) for IL1β, IL18, IL33 and the other ones.
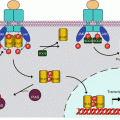
There are two IL1 receptors (IL1R1 and IL1R2) and two IL18 receptors (IL18R1 and IL18BP, for IL18-binding protein), but there is only one for IL33 (IL33R or ST2L, gene IL1RL1) and one for the group of IL36α, IL36β, IL37 and IL38 (IL36R, gene IL1RL2). IL1R1 contains a large cytoplasmic domain and is able to transduce appropriate signalling, whereas IL1R2 has lost the major part of this domain and cannot transduce any message; it behaves as a decoy receptor and can be removed from the membrane and adopt a soluble extracellular form; this is the same for IL18BP toward IL18. In addition to these receptors, there are accessory proteins (ILRAP), which are coreceptors able to form heterodimers with the primary receptors, constituting thus ternary complexes IL–ILR–ILRAP required for signal transduction. IL1α and β, IL33 and IL36 utilise IL1RAP, while IL18 utilises IL18RAP. The combinatory pattern of ligands and receptors of the IL1 family is presented Fig. 12.1a. Other ‘orphan’ receptors have also been identified. All these receptors, collectively called ILRs, contain, at the extracellular level, immunoglobulin-like domains and, for the active receptors, a domain called TIR (toll–interleukin receptor).
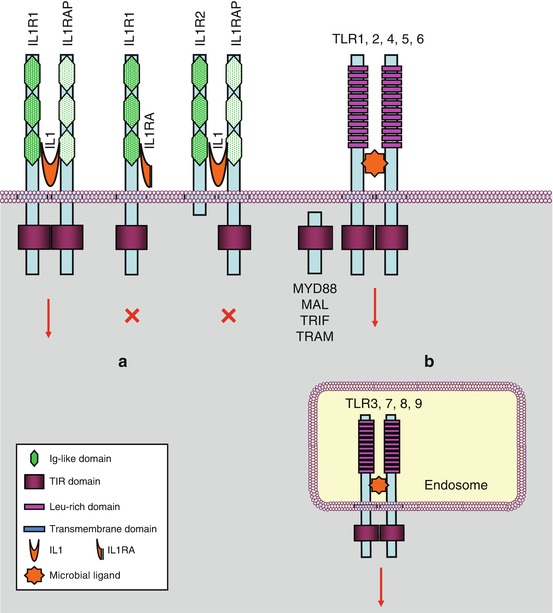

Fig. 12.1
Interleukin 1 receptors and toll-like receptors. (a) Interleukin 1 receptors (IL1R) transmit a signal (red arrow) when IL1 is bound to a complex IL1R1–IL1RAP (IL1 receptor accessory protein). IL1RA (IL1 receptor antagonist) cannot transmit a signal. IL1 binding on an IL1R2–IL1RAP complex cannot transmit a signal. (b) Toll-like receptors are localised on the plasma membrane or at the level of endosomes. They induce signalling when homodimerised, sometimes heterodimerised (TLR1 or 6 with TLR2)
IL1 family interleukins are basic mediators of immunity and inflammation. Most of them have a proinflammatory role, against which act the antagonistic ligands, IL1RN and IL36RN, as well as the decoy receptors IL1R2 and IL18BP. Caspase 1 activation is the initiating event of IL1 activation. This occurs at the level of supramolecular platforms, called inflammasomes, where adapter proteins enable the autoproteolytic activation of procaspase 1 into an active caspase, similarly as apoptosomes enable the activation of procaspase 9 (Chap. 18). The physiological roles of IL1 will not be described here; we will only mention the involvement of IL1β in autoimmune diseases, in which the inflammatory aspect is important and which are called auto-inflammatory diseases. Numerous strategies for blocking IL1, its activators and its effectors, have been developed in therapeutics.
12.2 Toll-Like Receptors and Their Ligands
Toll-like receptors (TLRs) have been originally discovered in Drosophila and then researched and identified in mammals. They are characterised by intracytoplasmic domains that are homologous to those of IL1 family receptors, but their extracellular domains are different: they contain leucine-rich domains and no immunoglobulin-like domains. The signalling pathways downstream toll-like and IL1 receptors are mostly common. There are nine TLRs (TLR1 to TLR9), among which five are present on the plasma membrane and four on endosomal membranes (Fig. 12.1b). Their activation results from dimerisation, generally homodimerisation, sometimes heterodimerisation as the one occurring between TLR2 and its partners TLR1 and TLR6.
The ligands able to activate TLRs are from extracellular or intracellular origin. These are essentially molecules of microbial origin, generically called PAMPs (pathogen–associated molecular patterns): lipopolysaccharides (LPS) of Gram-negative bacteria for TLR4; lipoproteins and lipoteichoic acid for TLR1, 2 and 6; flagellin for TLR5 and viral nucleic acid fragments, essentially RNAs, for the intracellular TLR3, 7, 8 and 9. Numerous endogenous ligands are also able to activate TLRs, mainly TLR2 and 4: heat-shock proteins (HSPs), HMGB1 (high mobility group box 1) protein, uric acid crystals, surfactants, glycosaminoglycans, S100A9 protein and extracellular matrix proteins, such as fibrinogen and fibronectin.
Without entering into the details of the physiological roles of TLRs in immunity and inflammation, one can mention that TLRs, at the surface of epithelial cells, are at the forefront of recognition of infectious agents. Their roles in the fight against infection are varied: they are able to induce an inflammatory reaction, to activate NADPH oxidase which generates reactive oxygen and nitrogen species, to recruit leukocytes and macrophages, to allow the activation of cytokines such as interleukin 12 and interferons (Chap. 4) or interleukin 1, etc.
12.3 Signal Transduction from TLRs and ILRs
12.3.1 General Aspects
Several signalling pathways can be opened from ILRs and TLRs: the MAP kinase pathways (ERK, p38, JNK, Chap. 2); the NFκB pathway, which is presented below; as well as the IRF3 (Interferon regulatory factor 3) pathway, which regulates the activity of cytokines (Chap. 4). NFκB activation occurs following the activation of most ILRs and TLRs, with the exception of TLR3; IRF3 activation mainly occurs downstream TLR3 receptor activation.
The signals generated by ILRs and TLRs are overall similar, because both receptor families contain a common TIR (toll–interleukin receptor) domain in their intracellular segment. TIR domains are also present in the sequence of a series of adapter proteins which are recruited by homophilic interactions. The MYD88 (myeloid differentiation primary response gene 88) protein is an adapter protein common to all ILRs and TLRs; other adapter proteins are specific for TLR2 and/or TLR4, especially MAL/TIRAP (MYD88 adapter–like/TIR domain-containing adapter protein), TRIF (TIR domain-containing adapter-inducing interferon β) and TRAM (TRIF-related adapter molecule). Receptor activation occurs through dimerisation and is followed by the recruitment of these proteins, which will in turn recruit cytoplasmic kinases, called IRAKs (IL1 receptor–associated kinases).
12.3.2 NFκB Pathway
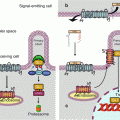
In the pathway leading to NFκB (nuclear factor of kappa light polypeptide gene enhancer in B-cells) downstream the activation of IL1R, TLR4 or the endosomal TLRs, the first step is the autophosphorylation of IRAK1, stimulated by IRAK4, which allows the recruitment of the adapter protein TRAF6 (TNF receptor-associated factor 6), a member of the TRAF family, and of another adapter protein, pellino 1 (gene PELI1), which together form the complex 1 (Fig. 12.2). The cytoplasmic constituents of complex 1 detach from the receptor and can interact with a membrane-bound MAP3 kinase (Chap. 2), TAK1 (TGFβ–activated kinase 1) (gene MAP3K7) and with two other adapter proteins, TAB1 and TAB2 (TGFβ–activated kinase 1 binding protein) to form complex 2. At the level of complex 2, comprising IRAK1, TRAF6, TAK1, TAB1 and TAB2, IRAK1 can phosphorylate TAK1, which allows the dissociation of this complex from the membrane and its release in the cytosol as complex 3 (which comprises thus TRAF6, TAK1, TAB1 and TAB2). TRAF6 is an E3 ubiquitin ligase (see Annex C) able to perform auto-ubiquitinylation; this does not lead it to the proteasome, but on the contrary enables it to activate complex 3. TAK1 can thus phosphorylate a serine/threonine kinase called IKK (IκB kinase), which is responsible for the formation of NFκB. As TAK1 is a MAP3 kinase, it can also phosphorylate the MAP2 kinases of the p38 and JNK pathways (Chap. 2).
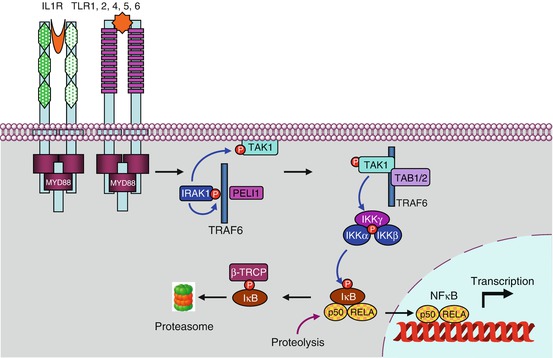

Fig. 12.2




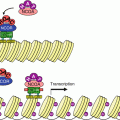
The main signalling pathway downstream TLRs and ILRs. After the intervention of an adapter protein (MYD88), IRAK1 can autophosphorylate and recruit other adapter proteins (TRAF6, PELI1) and form a complex. This complex can activate the membrane MAP3 kinase, TAK1, through phosphorylation by IRAK1 and form another complex after recruitment of the adapter proteins TAB1 and TAB2. This complex can leave the membrane, and TAK1 can phosphorylate IKK, which is comprised of two kinase subunits, IKKα and β, and a regulatory subunit, IKKγ or NEMO. IKK can then phosphorylate the proteins IκB, which leads them to proteasome. The transcription factors of the NFκB–REL family are released, and their nuclear localisation sequence unmasked; they can thus translocate into the nucleus and exert their function on target genes. In parallel to this canonical pathway, other modalities can lead to NFκB or IRF3 activation; they require other adapter proteins and other kinases for the phosphorylation of IκB proteins, but the general scheme remains the same
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree