22
Tolerance
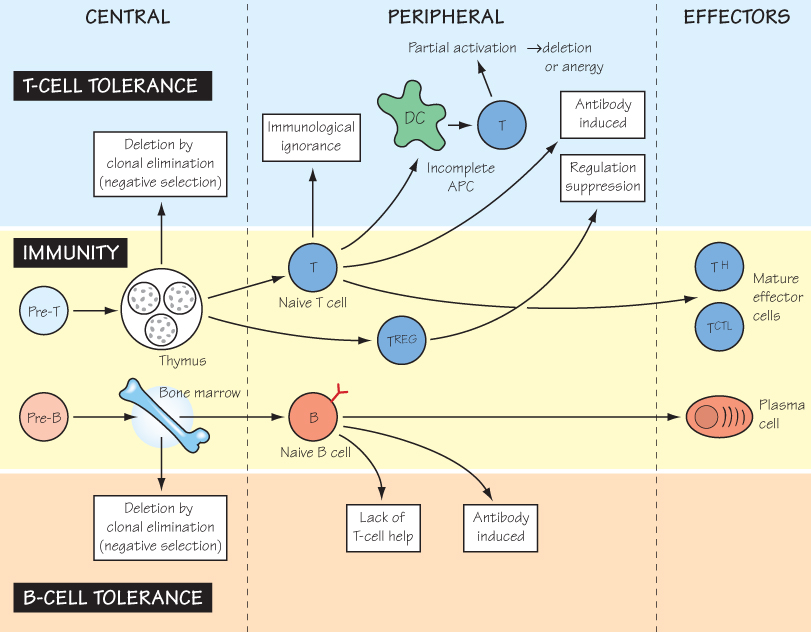
The evolution of recognition systems that initiate destruction of ‘non-self’ material obviously brings with it the need for safeguards to prevent damage to ‘self’. This is a particularly acute problem for the adaptive immune system, because the production of T-cell and B-cell receptors involves an element of random gene rearrangement (see Figs 12 and 13), and therefore lymphocytes with receptors directed at ‘self’ will inevitably emerge in each individual. Furthermore, ‘self’ for one individual is not always the same as ‘self’ for another. For example, people of blood group A have red cells that carry antigen A but make antibodies to blood group B, and vice versa. The AB child of an A father and a B mother inherits the ability to make both anti-B and anti-A antibodies but must not make either, i.e. it must be tolerant to A and B.
Adaptive immunity, both B and T cell, in fact protects itself against possible self-reactivity at several stages (as shown in the figure). It used to be assumed that elimination of potentially self-reactive clones (negative selection) was the basis of all unresponsiveness to self, but many other regulatory mechanisms are now recognized. Nevertheless, self-tolerance is not absolute, and in some cases failure may lead to self-destructive immune responses (see Fig. 38).
In certain circumstances, normally antigenic ‘non-self’ materials can trigger these safeguarding mechanisms, a state known as induced tolerance, which might be very undesirable in some infections but very useful in the case of an organ transplant. The mechanisms involved in induced tolerance are likely to be very similar to those that maintain self-tolerance. Note that tolerance is by definition antigen specific, and quite distinct from the non-specific unresponsiveness induced by damage to the immune system as a whole, which is instead described as immunodeficiency (see Fig. 33).
Clonal Elimination
A cornerstone of Burnet’s clonal selection theory (1959) was the prediction that lymphocytes were individually restricted in their recognition of antigen and that self-recognizing ones were eliminated early in life in the primary lymphoid organs. This is achieved for T cells by negative selection in the thymus (see Fig. 16), and for B cells in the bone marrow. Negative selection was first demonstrated convincingly for superantigens, such as those expressed by some mice endogenous retroviruses, because these delete a substantial proportion of T cells in the thymus. Neither B-cell nor T-cell deletion during development is complete, necessitating the existence of mechanisms of tolerance induction outside the primary lymphoid organs (peripheral tolerance).
Immunological Ignorance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




