and Paul M. Cinciripini1
(1)
Department of Behavioral Science, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Tobacco use is behind most preventable diseases with disabling consequences and death. These diseases are among the most serious, including cancer, cardiovascular diseases (brain strokes, cardiac infarcts, peripheral artery disease), and respiratory system diseases (emphysema, chronic infections). It is estimated that one-third of cancers are attributable to tobacco use and in theory can be prevented. Therefore, a comprehensive tobacco cessation program is a crucial element of successful survivorship and cancer prevention programs. Smoking cigarettes is the most common and deadliest method of consuming tobacco, and nicotine is the reinforcing substance in any tobacco use that with long-term exposure leads to dependence (addiction). Nicotine dependence involves biological, behavioral, and cognitive elements; an optimal approach to treatment for nicotine dependence should address each of these three dimensions. A comprehensive tobacco/smoking cessation program should include cognitive behavioral techniques, motivational interviewing approaches, and appropriate medications. Currently the medications approved by the US Food and Drug Administration for the treatment of nicotine dependence include nicotine replacement therapies, bupropion-SR (sustained release), and varenicline; these treatments can be used individually or in combination. Combining medications capitalizes on the synergy resulting from differing mechanisms of action.
Introduction
Cigarette smoking is the principal cause of preventable morbidity and mortality in the United States (US Centers for Disease Control and Prevention [CDC] 2010) and around the globe. In the United States alone, 443,000 deaths per year are attributable to cigarette smoking, according to the CDC; around the world, that number is estimated to be about six million deaths per year. Although tobacco use in general correlates with many cancers, cigarette smoking in particular is reported to be causally linked to at least 18 types of cancer. Smoking-related health care expenditures in the United States are estimated to be around $96 billion, and costs related to the accompanying loss in productivity are about $97 billion, resulting in an economic burden from smoking of about $193 billion per year (CDC 2012).
Approximately 12 million people are living with cancer in the United States (CDC 2012); lung cancer, ischemic heart disease, and chronic obstructive pulmonary disease constitute the three leading causes of smoking-attributable mortality. Smoking cigarettes accounts for the vast majority of tobacco use and addiction, as well as for the vast majority of nicotine dependence (for the purpose of this chapter we will use the term “tobacco addiction” interchangeably with “smoking cigarettes” or “nicotine dependence”). Therefore, treating tobacco addiction must be an essential component of any campaign to eradicate cancer, in particular because of the staggering statistics pointing to smoking as the cause of one out of every three deaths from cancer and as the cause of four out of five deaths from chronic obstructive pulmonary disease. Because the consequences of smoking take many years or decades to become apparent, declining smoking rates and increasing public health campaigns against tobacco will take years or even decades to make a dent in the current death toll.
Unfortunately, smoking cigarettes remains the leading cause of death in the United States even though cigarette use has declined substantially in United States and other industrialized nations. However, there is reason for hope, as evidenced by outcomes of public health programs in the state of California, where aggressive campaigns with provisions for treatment did ultimately decrease cigarette use, which is currently at around 15% in the state (CDC 2011). This is the second lowest smoking rate in the nation, after Utah (13%). Interestingly, this decrease in smoking in California was followed by a substantial reduction in lung cancer incidence within 5 years and thereafter. For 2009 the incidence of new lung cancer cases/year was at 60 and 78 per 100,000 for California versus the US respectively, while in 1999 that incidence was at 75 and 93 cases per 100,000 for California versus the US respectively. This provides concrete evidence at the population level of the causal relationship between smoking and lung cancer and between quitting smoking and decreased lung cancer incidence.
Overall, despite several public health campaigns, one-fifth of the US population (<20%) currently smokes cigarettes. Unfortunately, smoking rates are substantially higher among certain groups; rates increase gradually with lower education levels and lower income levels. Yet 70% of smokers, when asked, say they would like to quit, and 40% of current smokers have made at least one quit attempt of at least 24 hours in the previous year, although only 6% manage to maintain abstinence from cigarettes when they quit without assistance (US Department of Health and Human Services 2000). Evidence shows that the difficulty in maintaining abstinence after quitting, whether assisted or not, is strongly related to affective and cognitive dysfunction, which may persist for some time after the initial cessation. In addition, cravings for cigarettes after cessation can result in a slip to smoking (less than 24 hours of smoking), and those slips often lead to full relapse to regular smoking.
Biological and Behavioral Determinants of Nicotine Dependence
The Reward Pathway
Cigarettes contain nicotine, a highly addicting substance. Like most drugs that are used for prolonged periods, nicotine can lead to dependence (traditionally referred to as addiction) because it acts on and stimulates specific receptors. Because nicotine receptors are spread in most areas of the brain, the administration of nicotine leads to a rapid increase in dopamine release within the nucleus accumbens and the ventral tegmental area (the two main areas of the reward pathway). This stimulation typically starts within 10 seconds of smoking a cigarette. It has been established that natural rewards such as food consumption, social affiliation, and sexual activity, which are linked to survival of the individual or species, also activate these two central areas of the reward pathway within the brain. The reward pathway has projections to many areas of the brain; of particular importance are the projections from the nucleus accumbens and ventral tegmental area to the prefrontal cortex, the amygdala, and the olfactory tubercle (Fig. 15.1). Several other brain systems (neurotransmitters and pathways) are thought to be involved in the process of developing dependence to a substance, although dopamine is referred to as the final or common neurotransmitter in the reward pathway.
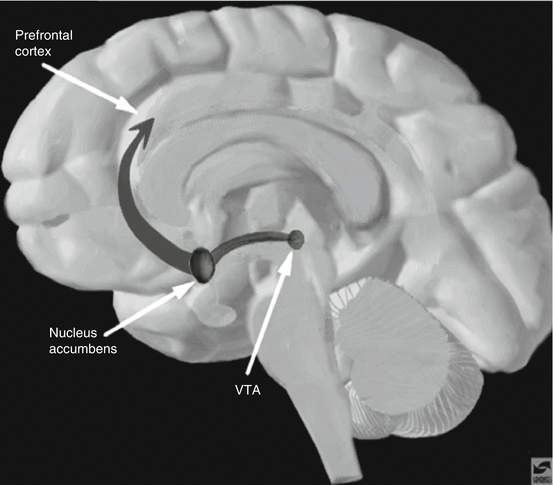

Fig. 15.1
The nucleus accumbens and ventral tegmental area (VTA) project to the prefrontal cortex as part of the reward pathway
Neuronal Adaptation
A pleasurable sensation from the activation of the reward pathway is associated with the acute use of a substance of abuse such as nicotine. However, repeated administration of a substance such as nicotine over months or years is likely to lead to increased tolerance, which in turn produces a state of withdrawal in the absence of the substance. Tolerance and withdrawal are the physiologic hallmarks of dependence and are thought to be the result of neuroadaptive effects occurring within the brain (Benowitz 2008). Interestingly, the chronic use of drugs of abuse and dependence (including nicotine) appears to result in a generalized decrease in dopaminergic neurotransmission. This decrease is likely to be a homeostatic response to the intermittent yet repetitive increases in dopamine induced by the frequent and sustained use of such drugs (Volkow et al. 2002).
Diagnosis of Nicotine Dependence
Because specific biological markers are absent, nicotine dependence is a clinical diagnosis. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5; American Psychiatric Association 2013), employs universal criteria for all substance dependence, including nicotine use disorder (formerly nicotine dependence). According to DSM-5, a substance use disorder is diagnosed when the patient meets two or more of the 11 total criteria within a 12-month period. The DSM-5 criteria offer ease of use for the clinician because of the universality of the criteria to all substances of dependence. However, because of their universality, the DSM-5 criteria are not specific to tobacco and therefore do not capture many of the particular aspects of tobacco use and nicotine dependence. This nonspecific categorization has led to the development of specific scales to quantify nicotine dependence. Traditionally, the Fagerström Test of Nicotine Dependence has been used, although recently the Wisconsin Inventory of Smoking Dependence Motives has become more accepted as a more comprehensive scale.
Smoking and Psychiatric Comorbidities
Smoking rates among individuals with no mental illness, past-month mental illness, and lifetime mental illness have been reported to be 22%, 34%, and 41%, respectively. These rates indicate that having a current mental disorder effectively doubles the chances of being a smoker. Furthermore, in a nationally representative sample, smokers who had a mental disorder in the past month were reported to consume 44% of all cigarettes smoked (Lasser et al. 2000). Smoking seems to be closely linked with several psychiatric comorbidities, including dependence on other substances, suggesting a shared biological pathway between nicotine dependence and these other psychiatric conditions. Evidence of co-occurrence of mental illness with smoking also highlights the importance of screening and treating mental health disorders among smokers, whether the co-occurrence is causal or a simple correlation. Treating these comorbid mental disorders would at least reduce the impact of the disorders on patients’ ability to quit smoking, and treating such disorders may increase patients’ resilience against relapsing to cigarette use. This is of particular importance among patients who are in remission from cancer (survivors) who relapse to or continue to smoke and are unable to quit because they may still be recovering from the emotional toll of cancer, which often leads to clinical depression or anxiety disorders.
Treatment for Tobacco Use
To achieve maximum benefits, the treatment approach for tobacco use disorder (nicotine dependence) must be comprehensive, because the disease itself has multiple components. Similarly, the approach must be ongoing or longitudinal because dependence is a chronic relapsing disorder. The essential components of a treatment program are psychosocial therapies and medications. Therapies such as cognitive behavioral therapy, motivational interviewing, skills building, and problem solving have been shown empirically to be effective.
First-line medications approved by the US Food and Drug administration (FDA) comprise three major categories: (1) nicotine replacement therapies (NRTs); (2) sustained-release bupropion (bupropion-SR), a nicotine receptor antagonist; and (3) varenicline (Chantix), a nicotine receptor partial agonist. The US Department of Health and Human Services updated the Clinical Practice Guideline for Treating Tobacco Use and Dependence (CPG-TTUD) in 2008 (Fiore et al. 2008). This guideline is evidence-based and is considered the standard of practice in providing treatment for tobacco and smoking cessation; it can be summarized in ten key recommendations (Table 15.1). Medications have a big impact on smoking cessation, reduction of cravings, and mitigation of nicotine withdrawal symptoms. NRTs, bupropion-SR, and varenicline are first-line therapies for nicotine dependence (Table 15.2), whereas nortriptyline (Pamelor) and clonidine (Catapres) are not approved by the FDA for this particular use and are considered second-line therapies owing to their side effect profiles.
Table 15.1
Ten key recommendations for tobacco and smoking cessation treatment programs
The overarching goal of these recommendations is that clinicians strongly recommend the use of effective tobacco dependence counseling and medication treatments to their patients who use tobacco, and that health systems, insurers, and purchasers assist clinicians in making such effective treatments available. |
1. Tobacco dependence is a chronic disease that often requires repeated intervention and multiple attempts to quit. Effective treatments exist, however, that can significantly increase rates of long-term abstinence. |
2. It is essential that clinicians and health care delivery systems consistently identify and document tobacco use status and treat every tobacco user seen in a health care setting. |
3. Tobacco dependence treatments are effective across a broad range of populations. Clinicians should encourage every patient willing to make a quit attempt to use the counseling treatments and medications in this Guideline. |
4. Brief tobacco dependence treatment is effective. Clinicians should offer every patient who uses tobacco at least the brief treatments shown to be effective in this Guideline. |
5. Individual, group, and telephone counseling are effective, and their effectiveness increases with treatment intensity. Two components of counseling are especially effective, and clinicians should use these when counseling patients making a quit attempt: |
Practical counseling (problem solving/skills training) |
Social support delivered as part of treatment |
6. Numerous effective medications are available for tobacco dependence, and clinicians should encourage their use by all patients attempting to quit smoking—except when medically contraindicated or with specific populations for which there is insufficient evidence of effectiveness (i.e., pregnant women, smokeless tobacco users, light smokers, and adolescents). |
Seven first-line medications (five nicotine and two non-nicotine) reliably increase long-term abstinence: bupropion-SR, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, nicotine patch, and varenicline. |
Clinicians also should consider the use of certain effective combinations of medications in this Guideline. |
7. Counseling and medication are effective when used individually for treating tobacco dependence. The combination of counseling and medication, however, is more effective than either treatment alone. Thus, clinicians should encourage all individuals making a quit attempt to use both counseling and medication. |
8. Telephone quitline counseling is effective with diverse populations and has broad reach. Therefore, both clinicians and health care delivery systems should ensure patient access to quitlines and promote quitline use. |
9. If a tobacco user currently is unwilling to make a quit attempt, clinicians should use the motivational treatments shown in this Guideline to be effective in increasing future quit attempts. |
10. Tobacco dependence treatments are both clinically effective and highly cost-effective relative to interventions for other clinical disorders. Providing coverage for these treatments increases quit rates. Insurers and purchasers should ensure that all insurance plans include the effective counseling and medication in this Guideline as covered benefits. |
Table 15.2
Dosage and availability of US Food and Drug Administration–approved pharmacologic agents for smoking cessation
Cessation agent | Dosage | Label indication and use | Availability in the United States | OR of efficacy (95% CI) |
|---|---|---|---|---|
Nicotine gum | 2 and 4 mg | 2 mg for ≤25 cigarettes per day and 4 mg for >25 cigarettes per day; minimum 8 pieces per day, maximum 20 pieces per day | OTC; traditional, mint, and orange flavors; generic available | 1.66 (1.52–1.81)a |
Nicotine patch | 21, 14, and 7 mg | ≥10 cigarettes per day: 21 mg for 6 weeks, then 14 mg for 2 weeks, then 7 mg for 2 weeks | OTC; clear and skin color; generic available | 1.81 (1.63–2.02)a |
Nicotine nasal spray | 10 mg/mL, 0.5 mg per squirt | 2 squirts (1 dose) per hour, minimum 8 doses per day, maximum 40 doses per day | Prescription only, 100 mg per bottle; no generic | 2.35 (1.63–3.38)a |
Nicotine oral inhaler | 10 mg per cartridge, 4 mg delivered | 6–16 cartridges per day up to 12 weeks, then gradual reduction for 12 weeks | Prescription only, 168 cartridges per box; no generic | 2.14 (1.44–3.18)a |
Nicotine lozenges
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|