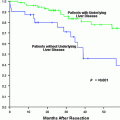
Fig. 1
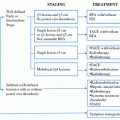
Treatment algorithm of patients with HCC based on serum bilirubin level and indocyanine green retention rate at 15 min. Adapted from Makuuchi et al. [8]
2.3 Assessment of the Future Liver Remnant
Evaluation of the volume of the FLR is the most reliable approach to predict outcome in patients who are candidates for major liver resection. Several methods have been described to evaluate the volume of the FLR. At MD Anderson Cancer Center, we calculate the estimated total liver volume (TLV) by using a formula that relies on the linear correlation between the TLV and body surface area (BSA), as follows:
TLV (cm3) = −794.41 + 1,267.28 × BSA (m2).
The standardized FLR (sFLR) is calculated as the ratio of the FLR divided by the estimated TLV. In a series of 301 patients without chronic liver disease or hepatic injury undergoing extended right hepatectomy, we showed that a sFLR of less than 20 % was a risk factor for postoperative liver insufficiency and 90-days postoperative mortality [9]. There is not yet agreement on the cutoff value for the sFLR volume requirement for patients with chronic liver disease. At MD Anderson, we consider that an sFLR of 40 % or more of the estimated total liver volume is necessary for patients with cirrhosis [10].
3 Methods to Improve Resectability of Hepatocellular Carcinoma
3.1 Portal Vein Embolization
In patients who are primarily not eligible for liver resection because of insufficient FLR volume, PVE has been recognized as a safe and effective method of inducing hypertrophy of the FLR. PVE is usually performed under fluoroscopic guidance and involves cannulation of the ipsilateral branch of the portal vein and embolization of the entire portal vein tree to be resected, using microparticles followed by coils or absolute ethanol. PVE induces atrophy of the embolized liver and a compensatory hypertrophy of the contralateral liver segments. This procedure has been shown to be safe and associated with low morbidity in large series of patients [11].
Contraindications to this approach are the presence of a portal vein thrombosis or severe portal hypertension.
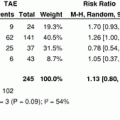
In a series of 301 patients, Kishi et al. [9] showed that patients with insufficient sFLR who achieved the cutoff volume requirement (>20 %) after PVE had outcomes similar to those of patients who had sufficient sFLR without PVE (Fig. 2). Furthermore, two studies have demonstrated that the magnitude of the hypertrophy reflects the regenerative capacity of the liver. In a prospective study of 55 patients, Farges et al. [12] showed that the use of PVE decreased the morbidity rate in patients with cirrhosis. In this study, the authors observed that mortality rate was increased in patients whose liver did not show hypertrophy of the FLR after PVE. In a series of 112 consecutive patients undergoing PVE before hepatectomy, Ribero et al. [13] demonstrated that the postoperative complications rate, including liver-related complications, was higher in patients whose liver showed a degree of hypertrophy less than 5 % (Fig. 3).
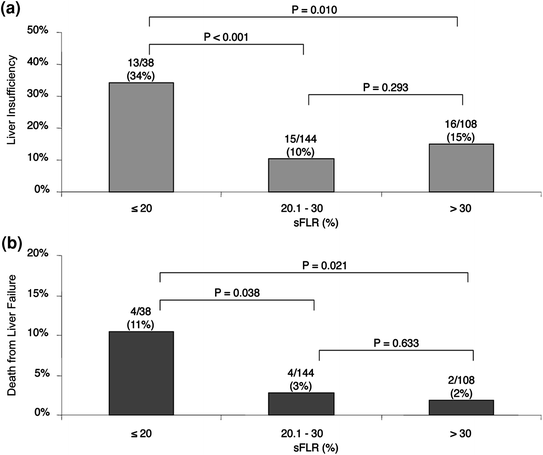
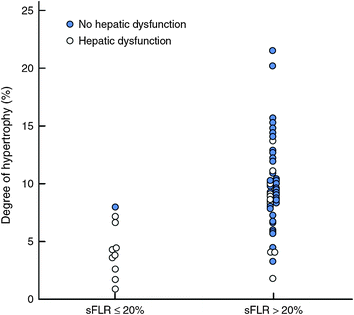

Fig. 2
Incidences of postoperative liver insufficiency (a) and death due to liver failure (b) according to preoperative sFLR. Adapted from Kishi et al. [9]

Fig. 3
Incidence of hepatic dysfunction according to degree of hypertrophy, stratified by standardized future liver remnant (sFLR). Adapted from Ribero et al. [13]
In patients with an anticipated small FLR, then, PVE is useful for two reasons: liver hypertrophy after PVE reflects the regenerative capacity of the liver, and increase of the FLR volume may expand the indications for liver resection in patients with chronic liver disease whose tumor was initially deemed unresectable because of a small liver remnant.
3.2 Preoperative Transarterial Chemoembolization
The potential benefit of TACE before liver resection in patients with HCC is twofold: (1) downsizing of the tumor with the aim of performing a more limited resection, and (2) decreasing the recurrence rate after resection. Preoperative TACE has been assessed in several retrospective series, and most of these studies failed to show a benefit associated with this strategy in HCC patients [14, 15]. Some of these studies even concluded that TACE could be associated with worse outcome, including increased intraoperative difficulties [14], increased postoperative morbidity, and lower chance of long-term survival [16]. The absence of clear benefit associated with preoperative TACE has been confirmed in a prospective randomized trial on 108 patients with resectable HCC 5 cm or greater in size. In this study, 10 % of the patients receiving preoperative TACE could not undergo resection because of extrahepatic disease progression or liver failure. Patients in the preoperative TACE group had a longer operative procedure related to greater surgical difficulties due to local adherence and inflammation. However, postoperative morbidity and mortality were similar in both groups, as were disease-free and overall survival rates, demonstrating the lack of benefit of preoperative TACE in patients with resectable HCC [17].
3.3 Sequential Chemoembolization and Portal Vein Embolization
The sequential use of TACE and PVE has been evaluated: first to improve disease control in HCC patients and second to promote hypertrophy of the FLR in patients with chronic liver disease. This approach has been assessed in three retrospective series. Aoki et al. [18] first reported the feasibility of this approach, demonstrating low morbidity in 17 patients with HCC. The authors showed 22 ± 4 % hypertrophy of the nonembolized segments within 2 weeks after sequential TACE+PVE, allowing subsequent major hepatic resection in 16 patients. No liver failure occurred after surgery and 5-year overall and disease-free survival rates were 56 and 47 %, respectively. Ogata et al. [19] compared 36 patients undergoing preoperative PVE (n = 18) or sequential TACE+PVE (n = 18). The authors showed that the mean increase in percentage FLR volume was significantly higher in the TACE+PVE group than in the PVE group (mean 12 ± 5 versus 8 ± 4 %); P = 0.022). The rate of insufficient liver hypertrophy following the procedure was significantly higher in patients undergoing only PVE than in those undergoing sequential TACE+PVE (13 versus 5, respectively; P = 0.044). Furthermore, the incidence of complete tumor necrosis was significantly higher in the TACE+PVE group (15 of 18 versus 1 of 18; P < 0.001), as was the 5-year disease-free survival rate (37 versus 19 %; P = 0.041). The oncologic benefit of this approach was confirmed in a large series of 135 patients who underwent resection after sequential TACE+PVE (n = 71) or after PVE alone (n = 64) [20]. In this series, patients undergoing resection after sequential TACE+PVE had better overall and recurrence-free survival rates than those undergoing resection after PVE alone (P = 0.028 and P = 0.001, respectively) (Fig. 4). The sequential use of TACE+PVE increases the number of surgical candidates by promoting liver hypertrophy. By inducing massive tumor necrosis, moreover, this procedure improves disease control in patients undergoing resection of HCC.
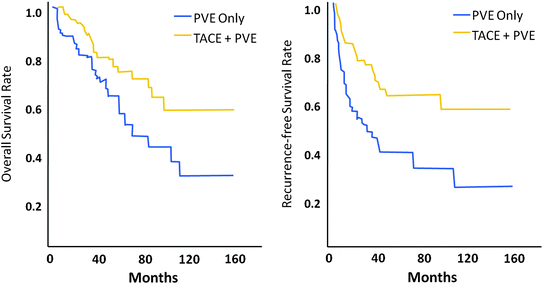

Fig. 4
Overall and disease-free survival rates after PVE+TACE and PVE only. Adapted from Yoo et al. [20]
3.4 Perspectives
Antitumor agents such as sorafenib and bevacizumab have been tested in a palliative setting in patients with inoperable HCC. Sorafenib has already been shown to prolong survival in unresectable HCC in two large randomized controlled trials [21, 22]. No study has evaluated the role of these therapies as an adjunct to surgery, either to limit the risk of disease recurrence or to render unresectable HCC resectable. In the future, these drugs and others may be evaluated in combination with surgery to increase the number of patients who have the opportunity for a curative approach.
Transarterial radioembolization has been shown to be a valuable alternative to TACE in patients with portal vein thrombosis [23]. This procedure is generally well tolerated and could also be evaluated as an adjunct to surgery in these patients.
4 Indications and Outcomes After Resection of Advanced Hepatocellular Carcinoma
4.1 Large HCC
Large tumor size (>5 cm) is a contraindication for OLT and is not an indication for ablation; liver resection remains the sole treatment option when the lesion appears to be safely resectable [24]. In some centers, large tumor size, which is generally associated with an increased risk of vascular invasion, is still considered a contraindication to resection in HCC patients [25]. Recent data suggest, however, that patients with large tumors should be considered for surgical resection. Young et al. [26] reported good oncologic outcome in 42 patients with HCC larger than 10 cm, with 5-year overall and disease-free survival rates of 45 and 43 %, respectively. In a large multicenter series of 300 patients with HCC larger than 10 cm, the reported perioperative mortality was 5 %, while the 5-year overall survival rate was 26.9 %. Patients with a solitary large HCC without vascular invasion had a 5-year survival rate of 40–45 % [27]. Tumor size itself should not be regarded as a contraindication to resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree