Graves’ disease
Graves’ disease is caused by autoantibodies that stimulate the TSH receptor and hence thyroid hormone synthesis and secretion, and thyroid growth (causing a diffuse goitre). Possible precipitating and predisposing factors include genetic susceptibility (suggested by an association with certain alleles of CTLA-4 and HLA) and environmental factors such as infection.
The mechanisms that may be involved in the pathogenesis of Graves’ hyperthyroidism include molecular mimicry (similarity between some infectious/exogenous antigens and human proteins) and thyroid cell expression of HLA class II molecules (which may act as antigen-presenting cells to initiate an autoimmune response).
Patients with Graves’ disease may have a personal or family history of other autoimmune disorders such as vitiligo, alopecia areata, pernicious anaemia, type 1 diabetes mellitus, myasthenia gravis or coeliac disease.
Toxic multinodular goitre and toxic adenoma
These are the result of focal and/or diffuse hyperplasia of thyroid follicular cells whose function is independent of regulation by TSH. Between 20% and 80% of toxic adenomas and some nodules of toxic multinodular goitres have somatic mutations of the TSH receptor gene that confer autonomous hyperactivity.
Thyroiditis
Thyroiditis (e.g. subacute viral, postpartum) can result in thyrotoxicosis by the release of preformed thyroid hormones from a damaged thyroid gland into the circulation.
Subacute (de Quervain’s) viral thyroiditis presents initially with thyrotoxicosis followed by hypothyroidism several weeks later. Recovery of normal thyroid function occurs 3–6 months later, but 10% of patients may have late relapses. A similar but painless thyroiditis may occur 3–6 months after delivery (postpartum thyroiditis) possibly due to the exacerbation of a previously subclinical autoimmune thyroiditis. The thyrotoxic phase lasts for about 1–4 weeks.
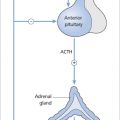
Secondary hyperthyroidism
Secondary hyperthyroidism due to a TSH-secreting pituitary tumour is very rare. A similar biochemical picture (high free T4/T3 and normal or high TSH) may be seen in the uncommon ‘thyroid hormone resistance syndrome’ (see below).
Amiodarone
Amiodarone (an iodine-containing antiarrhythmic drug) may affect thyroid function in several ways. Amiodarone inhibits the conversion of T4 to T3 and results in a high or high-normal free T4, low-normal free T3 and initially high TSH that normalizes within 2–3 months. In addition, amiodarone may cause both hypothyroidism (see Chapter 2) and thyrotoxicosis.
In amiodarone-induced thyrotoxicosis, clinical manifestations are often masked by the drug’s beta-blocking activity. Patients may present with atrial arrhythmias, exacerbation of ischaemic heart disease or heart failure, unexplained weight loss, restlessness or low-grade fever. There are two types of amiodarone-induced thyrotoxicosis. However, some patients may have a mixture of both types:
- Type 1 thyrotoxicosis is caused by amiodarone’s high iodine content, which provides the substrate for excessive thyroid hormone synthesis in patients with a previously silent multinodular goitre.
- Type 2 thyrotoxicosis is due to a direct toxic effect of the drug on the thyroid gland, resulting in a destructive thyroiditis and the release of preformed T4 and T3.
Subclinical hyperthyroidism
This condition may be endogenous (due to the same conditions that cause overt hyperthyroidism) or due to excess exogenous T4.
Clinical presentations
Clinical presentations of thyrotoxicosis are summarized in Box 3.2.
Examination of the neck may reveal a diffusely enlarged goitre (90% of patients with Graves’ disease), a multinodular goitre or a solitary nodule. A diffuse goitre may also be seen in painless thyroiditis and TSH-secreting pituitary tumours. Subacute (de Quervain’s) thyroiditis presents with a small tender goitre, and patients may have had a preceding flu-like illness.
Clinical signs specific to Graves’ disease include ophthalmopathy, pretibial myxoedema and thyroid acropachy. These are mediated by different autoantibodies that may co-exist in Graves’ disease.
Graves’ ophthalmopathy
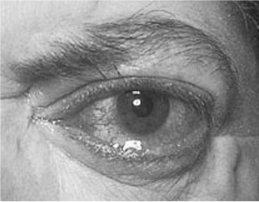
Graves’ ophthalmopathy (Fig. 3.1) may be clinically obvious in 20–25% of patients with Graves’ hyperthyroidism at the time of diagnosis of the hyperthyroidism. It is more common in females. Many more (>90%) may have evidence of ophthalmopathy on computed tomography/magnetic resonance imaging (CT/MRI) of the orbits. Around 10% of patients with Graves’ ophthalmopathy do not have Graves’ disease; they may have autoimmune hypothyroidism or thyroid autoantibodies.
Graves’ ophthalmopathy may present with periorbital oedema, conjunctival oedema (chemosis) and injection, grittiness, corneal ulceration, proptosis (60%), ophthalmoplegia and diplopia (40%), retrobulbar pain or pain on eye movement, and optic nerve compression (6%), which may result in impaired visual acuity or visual field defects. Graves’ ophthalmopathy may be unilateral in 15% of patients.
Pathogenesis involves activated T cell cytokines and TSH receptor antibodies that activate TSH receptors on fibroblasts and adipocytes. This sets off an inflammatory process and causes the secretion of hydrophilic glycosaminoglycans, resulting in an increased retro-orbital volume.
Patients with thyrotoxicosis due to any cause may have lid retraction and lid lag (sclera visible above the iris as the patient looks downward) caused by sympathetic overactivity, possibly mediated by increased beta-adrenergic receptors.
Figure 3.1 Graves’ ophthalmopathy.
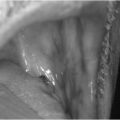
Pretibial myxoedema
Pretibial myxoedema (Fig. 3.2) is specific to Graves’ disease. It is seen in up to 2% of patients and results from an accumulation of hydrophilic glycosaminoglycans secreted by fibroblasts in the dermis. Pretibial myxoedema is characterized by raised, pigmented, orange-peel textured nodules or plaques on the anterior aspect of the leg or the dorsum of the foot. They are usually asymptomatic, but may be pruritic or painful.
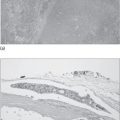
Thyroid acropachy
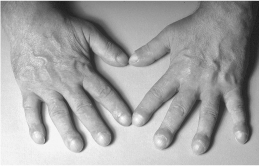
Thyroid acropachy (Fig. 3.3) is seen in fewer than 1% of the patients with Graves’ disease and resembles clubbing. It is due to periosteal new bone formation in the phalanges.
Figure 3.2 Pretibial myxoedema.
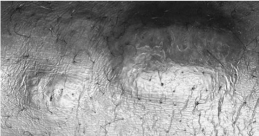
Figure 3.3 Thyroid acropachy.
Thyroid storm
A thyroid storm (‘thyrotoxic crisis’) may present with:
- fever, sweating
- cardiovascular symptoms: tachyarrhythmias, cardiac failure
- neurological symptoms: agitation, delirium, seizure, coma
- gastrointestinal symptoms: diarrhoea, vomiting, jaundice.
It has an untreated mortality of 50%. It may be precipitated by thyroid surgery, radio-iodine, iodinated contrast agents, withdrawal of thionamides (antithyroid drugs) and acute illnesses such as infection, stroke, diabetic ketoacidosis or trauma.
Investigations
Thyroid function tests
- In primary hyperthyroidism, thyroid function tests show suppressed serum TSH and high free T4 and or free T3.
- In secondary hyperthyroidism

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree