Surgery remains the most important effective treatment for differentiated (DTC) and medullary thyroid cancer (MTC). Radioactive iodine (RAI) is another important treatment but is reserved only for DTC whose disease captures RAI. Once patients fail primary therapy, observation is often recommended, as most DTC and MTC patients will have indolent disease. However, in a fraction of patients, systemic therapy must be considered. In recent decades 4 systemic therapies have been approved by the United States FDA for DTC and MTC. Sorafenib and lenvatinib are approved for DTC and vandetanib and cabozantinib for MTC. Anaplastic thyroid cancer (ATC) is a rare and rapidly progressive form of thyroid cancer with a very high mortality rate. Treatment of ATC remains a challenge. Most patients are not surgical candidates at diagnosis due to advanced disease. External beam radiation and radiosensitizing radiation are the mainstay of therapy at this time. However, exciting new drugs and approaches to therapy are on the horizon but it will take a concerted, worldwide effort to complete clinical trials in order to find effective therapies that will improve the overall survival for this devastating disease.
Key points
- •
Surgery remains the treatment of choice for differentiated thyroid cancer (DTC) and medullary thyroid cancer (MTC), with tyrosine kinase inhibitors reserved for symptomatic or rapidly progressive disease not amenable to surgery or other targeted therapies.
- •
Four multikinase inhibitors are US Food and Drug Administration approved for thyroid cancer: sorafenib and lenvatinib for DTC and vandetanib and cabozantinib for MTC.
- •
Anaplastic thyroid cancer (ATC) is a rare, highly aggressive, and lethal malignancy with median survival of less than 6 months. Initial evaluation and management require a rapid, coordinated, multidisciplinary team approach.
- •
Effective systemic therapies for ATC are lacking. Future improvement in outcomes will require the identification of driver genetic abnormalities or other aberrancies in the tumor microenvironment that can be targeted with novel agents.
Introduction
Thyroid cancer is the most common endocrine malignancy. Despite an increase in incidence in thyroid cancer, death rates have not changed significantly. There are 3 major types of thyroid cancers:
- •
Differentiated thyroid cancer (DTC) accounts for more than 90% of all thyroid cancer cases. DTC is derived from epithelial thyroid cells and includes papillary thyroid cancer (PTC), follicular thyroid cancer, Hürthle cell and poorly differentiated thyroid cancer (PDTC; Fig. 1 ) histologies. In theory, these types of thyroid cancers should be able to concentrate iodine, thus, in contrast with the other thyroid cancers, these types are treated with radioactive iodine (RAI). The prognosis for patients with well-differentiated DTC is generally very good, with a survival rate of more than 90%. PDTC represents intermediate entities in the progression of DTC to anaplastic thyroid cancer (ATC).
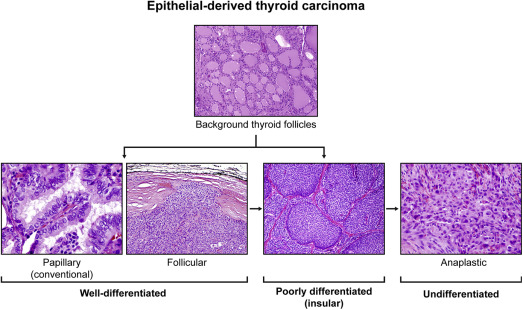
Fig. 1
Epithelial-derived thyroid cancer histologies. The most common types of thyroid cancer are derived from the epithelial thyroid cancer cells and includes papillary, follicular, poorly differentiated, and anaplastic thyroid cancers (hematoxylin-eosin, original magnification: top panel ×100; bottom panel ×400, ×100, ×100, and ×200).
- •
ATC is a rare type of thyroid cancer that is also derived from epithelial thyroid cells. It accounts for less than 2% of thyroid cancers and its incidence is approximately 500 cases/year in the United States. Unlike DTC and medullary thyroid cancer (MTC), ATC is one of the most aggressive malignancies in humans, with a median survival of less than 6 months. Patients often present with a rapidly enlarging neck mass associated with compressive symptoms (dyspnea, dysphagia) and pain. At diagnosis, more than one-third of patients have extrathyroidal extension and/or regional nodal metastases, whereas distant metastases are present in more than 40%. ATC can develop de novo or can derive from a thyroid cancer that is well differentiated. Morphologically ATC is undifferentiated, growing as sheets of cells without organization, with pleomorphism and high-grade features, including mitoses and necrosis ( Fig. 2 ). Diagnosis can be difficult because the cells usually have lost thyroid and epithelial cell-specific markers ( Table 1 ). In addition, there is a broad differential diagnosis on biopsy, and metastases to the thyroid and thyroid lymphoma must be excluded.
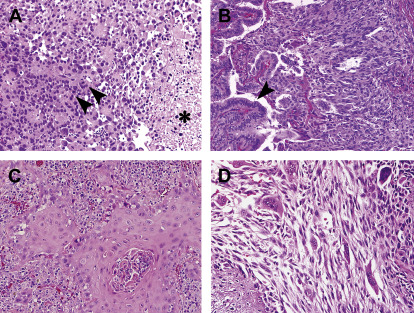
Fig. 2
Morphologic features and histologic variability in anaplastic thyroid carcinoma. ( A ) Discohesive, pleomorphic, and mitotically active tumor cells ( arrowheads ) are often associated with necrosis ( asterisk ). ( B ) Coexisting ATC ( right side , spindled cells) with a well-differentiated papillary thyroid carcinoma ( arrowhead ) showing organized vascular papillae lined by tumor cells. ( C ) Squamous morphology with glassy cells in cohesive nests surrounded by prominent inflammation. ( D ) Variable morphology within the same tumor, including spindled, giant, and epithelioid tumor cells all representing anaplastic carcinoma (hematoxylin-eosin, original magnification ×200).
Table 1
Histologic differential diagnosis and immunophenotype for anaplastic thyroid carcinoma (ATC)
Immunohistochemical Analysis
CKs
TTF1
Thyroglobulin
PAX8
Other
ATC
+ (75%) d
+ (5%–18%)
+ (1%–15%) (8%)
+ (30%–70%)
p53 (50%–75%)
Spindled Pattern
MTC
+
+
−
−
Synaptophysin, chromogranin, calcitonin c
True sarcoma (rare)
−
−
−
−
Various but not specific/differentiating
Epithelioid Pattern
Solid papillary thyroid carcinoma
+
+ (98%)
+ (>90%)
+ (100%)
—
Solid follicular thyroid carcinoma
+
+
+
+
—
PDTC
+
+ Variable
Variable/weak
+
—
Lymphoma
−
−
−
− a
CD45, various b
Metastasis to thyroid
—
—
—
—
—
Renal, GYN, GU, thymic
+
−
−
+
Various
Lung
+
+
−
−
—
Melanoma
− (Rare +)
−
−
—
Melan A, Tyrosinase, HBME-1
Neuroendocrine carcinoma
+
+ In some sites
−
−
Synaptophysin, chromogranin
Squamoid Pattern
SCC from adjacent H&N site
+, usually CK5/6 d
−
−
−
—
Metastatic SCC from another site
+, usually CK5/6 d
−
−
−
—
Abbreviations: CK, cytokeratin; H&N, head and neck; SCC, squamous cell carcinoma.
a Caution: some pax8 antibodies cross react with PAX5, which may be expressed on lymphoma cells.
b Immuno pattern varies with type of lymphoma.
c Calcitonin is not specific for MTC and occasionally is expressed in neuroendocrine carcinomas from other anatomic sites.
d Cytokeratin 5/6 may be expressed in ATC with squamoid differentiation; does not help with differentiating squamoid pattern tumors.
- •
MTC comprises approximately 1% to 2% of all thyroid cancers in the United States. Despite its rarity, this is a well-characterized neuroendocrine tumor ( Fig. 3 ). MTC arises from the parafollicular calcitonin-producing cells in the thyroid (C cells) and can occur sporadically (75%) or in a hereditary (25%) form associated with multiple endocrine neoplasia syndrome (MEN) types 2A and 2B. In patients with a palpable thyroid mass, 70% have cervical lymph node metastases and 10% to 15% have distant metastases. Based on the Surveillance, Epidemiology, and End Results database from 1973 to 2002, the 10-year survival rates for patients with MTC with localized, regional, and distant disease were 96%, 76%, and 40%, respectively.
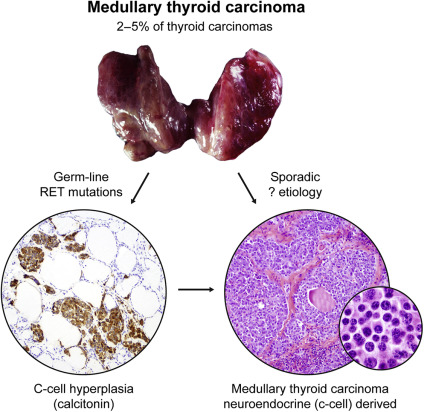
Fig. 3
Medullary thyroid cancer (MTC) histology. MTCs are neuroendocrine tumors and are derived from the neuroendocrine C cells in the thyroid gland. These tumors may be hereditary (caused by germline RET mutations) or sporadic (Calcitonin, original magnification ×100; hematoxylin-eosin, original magnification ×100 and ×400).
Introduction
Thyroid cancer is the most common endocrine malignancy. Despite an increase in incidence in thyroid cancer, death rates have not changed significantly. There are 3 major types of thyroid cancers:
- •
Differentiated thyroid cancer (DTC) accounts for more than 90% of all thyroid cancer cases. DTC is derived from epithelial thyroid cells and includes papillary thyroid cancer (PTC), follicular thyroid cancer, Hürthle cell and poorly differentiated thyroid cancer (PDTC; Fig. 1 ) histologies. In theory, these types of thyroid cancers should be able to concentrate iodine, thus, in contrast with the other thyroid cancers, these types are treated with radioactive iodine (RAI). The prognosis for patients with well-differentiated DTC is generally very good, with a survival rate of more than 90%. PDTC represents intermediate entities in the progression of DTC to anaplastic thyroid cancer (ATC).
Fig. 1
Epithelial-derived thyroid cancer histologies. The most common types of thyroid cancer are derived from the epithelial thyroid cancer cells and includes papillary, follicular, poorly differentiated, and anaplastic thyroid cancers (hematoxylin-eosin, original magnification: top panel ×100; bottom panel ×400, ×100, ×100, and ×200).
- •
ATC is a rare type of thyroid cancer that is also derived from epithelial thyroid cells. It accounts for less than 2% of thyroid cancers and its incidence is approximately 500 cases/year in the United States. Unlike DTC and medullary thyroid cancer (MTC), ATC is one of the most aggressive malignancies in humans, with a median survival of less than 6 months. Patients often present with a rapidly enlarging neck mass associated with compressive symptoms (dyspnea, dysphagia) and pain. At diagnosis, more than one-third of patients have extrathyroidal extension and/or regional nodal metastases, whereas distant metastases are present in more than 40%. ATC can develop de novo or can derive from a thyroid cancer that is well differentiated. Morphologically ATC is undifferentiated, growing as sheets of cells without organization, with pleomorphism and high-grade features, including mitoses and necrosis ( Fig. 2 ). Diagnosis can be difficult because the cells usually have lost thyroid and epithelial cell-specific markers ( Table 1 ). In addition, there is a broad differential diagnosis on biopsy, and metastases to the thyroid and thyroid lymphoma must be excluded.
Fig. 2
Morphologic features and histologic variability in anaplastic thyroid carcinoma. ( A ) Discohesive, pleomorphic, and mitotically active tumor cells ( arrowheads ) are often associated with necrosis ( asterisk ). ( B ) Coexisting ATC ( right side , spindled cells) with a well-differentiated papillary thyroid carcinoma ( arrowhead ) showing organized vascular papillae lined by tumor cells. ( C ) Squamous morphology with glassy cells in cohesive nests surrounded by prominent inflammation. ( D ) Variable morphology within the same tumor, including spindled, giant, and epithelioid tumor cells all representing anaplastic carcinoma (hematoxylin-eosin, original magnification ×200).
Table 1
Histologic differential diagnosis and immunophenotype for anaplastic thyroid carcinoma (ATC)
Immunohistochemical Analysis
CKs
TTF1
Thyroglobulin
PAX8
Other
ATC
+ (75%) d
+ (5%–18%)
+ (1%–15%) (8%)
+ (30%–70%)
p53 (50%–75%)
Spindled Pattern
MTC
+
+
−
−
Synaptophysin, chromogranin, calcitonin c
True sarcoma (rare)
−
−
−
−
Various but not specific/differentiating
Epithelioid Pattern
Solid papillary thyroid carcinoma
+
+ (98%)
+ (>90%)
+ (100%)
—
Solid follicular thyroid carcinoma
+
+
+
+
—
PDTC
+
+ Variable
Variable/weak
+
—
Lymphoma
−
−
−
− a
CD45, various b
Metastasis to thyroid
—
—
—
—
—
Renal, GYN, GU, thymic
+
−
−
+
Various
Lung
+
+
−
−
—
Melanoma
− (Rare +)
−
−
—
Melan A, Tyrosinase, HBME-1
Neuroendocrine carcinoma
+
+ In some sites
−
−
Synaptophysin, chromogranin
Squamoid Pattern
SCC from adjacent H&N site
+, usually CK5/6 d
−
−
−
—
Metastatic SCC from another site
+, usually CK5/6 d
−
−
−
—
Abbreviations: CK, cytokeratin; H&N, head and neck; SCC, squamous cell carcinoma.
a Caution: some pax8 antibodies cross react with PAX5, which may be expressed on lymphoma cells.
b Immuno pattern varies with type of lymphoma.
c Calcitonin is not specific for MTC and occasionally is expressed in neuroendocrine carcinomas from other anatomic sites.
d Cytokeratin 5/6 may be expressed in ATC with squamoid differentiation; does not help with differentiating squamoid pattern tumors.
- •
MTC comprises approximately 1% to 2% of all thyroid cancers in the United States. Despite its rarity, this is a well-characterized neuroendocrine tumor ( Fig. 3 ). MTC arises from the parafollicular calcitonin-producing cells in the thyroid (C cells) and can occur sporadically (75%) or in a hereditary (25%) form associated with multiple endocrine neoplasia syndrome (MEN) types 2A and 2B. In patients with a palpable thyroid mass, 70% have cervical lymph node metastases and 10% to 15% have distant metastases. Based on the Surveillance, Epidemiology, and End Results database from 1973 to 2002, the 10-year survival rates for patients with MTC with localized, regional, and distant disease were 96%, 76%, and 40%, respectively.
Fig. 3
Medullary thyroid cancer (MTC) histology. MTCs are neuroendocrine tumors and are derived from the neuroendocrine C cells in the thyroid gland. These tumors may be hereditary (caused by germline RET mutations) or sporadic (Calcitonin, original magnification ×100; hematoxylin-eosin, original magnification ×100 and ×400).
Genetics of thyroid cancer
In the last decade, molecular diagnostics have improved the care of patients with thyroid cancer. The Ras-Raf-MEK-MAP-ERK (MAPK) kinase signaling pathway plays a key role in development of thyroid cancer. Several studies have reported a high frequency of genetic alterations involved in the MAPK and phosphatidylinositol 3 kinase (PI3K) pathway ( Table 2 ):
- •
BRAF , RAS , PTEN mutations in DTC and ATC. Molecular alterations of ATC overlap with DTC and commonly have additional mutations in p53, beta-catenin, and PIK3CA.
- •
RET fusions in DTC.
- •
TERT promoter mutations in more aggressive, less-differentiated PTCs.
- •
Somatic RET , RAS mutations in MTC.
- •
Patients with MEN2A and MEN2B have a RET germline mutation that serves as the oncogenic basis of their MTC.
| Genes | Molecular Alteration | FA (%) | PTC Conventional (%) | PTC FV (%) | FC (%) | PD (%) | ATC (%) | MTC (%) |
|---|---|---|---|---|---|---|---|---|
| BRAF | Mutations; rare fusions | 0 | 71 | 20 | 0 | 15 | 25 | — |
| RAS a | Mutations | 30 | 7 | 35 | 45 | 30 | 50 | — |
| PAX8/PPARg | Fusion | 10 | 0 | 2 | 35 | 0 | 0 | 0 |
| RET/PTC b | Fusion | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| RET | Mutations | 0 | 0 | 0 | 0 | 0 | 0 | 50 c |
| p53 | Mutations | 0 | Rare | 0 | Rare | 30 | 65 | — |
| b-catenin | Mutations | 0 | Rare | 0 | 0 | 25 | 65 | — |
| EIF1AX | Mutations | Unk | 0 | Rare | Unk | Unk | Unk | Unk |
| ETV6-7/NTRK3 | Fusion | Unk | 0 | Rare | Unk | Unk | Unk | Unk |
| ALK | Fusion | Unk | 0 | Rare | Unk | Rare | Rare | Unk |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





