and Mouhammed Amir Habra1
(1)
Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Thyroid cancer survivors enjoy long life expectancy but may experience lifelong complications attributable to surgery, radioactive iodine therapy, or thyroid hormone suppression. The importance of these potential complications increases over time as the active initial cancer therapy is completed and the acute threat of the malignancy ebbs. Accordingly, a comprehensive examination of individuals treated for thyroid cancer should be undertaken as soon as possible to detect and correct problems and to improve quality of life in these patients. The Thyroid Cancer Survivorship Clinic at MD Anderson has been developed to provide an environment in which thyroid cancer survivors can be evaluated and potential future effects of cancer and cancer therapy can be addressed. In addition, providing this service can facilitate development of evidence-based guidelines for treatment goals and long-term surveillance.
Introduction
Awareness is growing that the life trajectory of individuals diagnosed with and treated for cancer can extend far beyond the often limited duration of active therapy. Thyroid cancer is the most common endocrine malignancy and is currently the fifth most common malignancy in women. Differentiated thyroid carcinoma, which includes papillary and follicular thyroid carcinoma, represents almost 95% of all cases of thyroid cancer. Medullary thyroid carcinoma, primary thyroid lymphoma, and anaplastic thyroid carcinoma represent about 5% of all cases of thyroid cancer. Recent projections estimate that 1,660,290 individuals will be newly diagnosed with cancer in the United States in 2013; of these, 60,220 individuals will be diagnosed with thyroid cancer but only 1,850 thyroid cancer-related deaths are expected to occur during the same period (Siegel et al. 2013). The number of patients with thyroid cancer is growing because of increasing incidence of the disease and improved survival durations of most patients with thyroid cancer. The overall 10-year survival rate for patients with differentiated thyroid carcinoma is about 90–95%, making this disease very attractive for the development of a survivorship model. Figure 14.1 illustrates the incidence of thyroid cancer between 1990 and 2011 in men and women as well as total mortality in both sexes. No guidelines have been formally established for thyroid cancer survivors and their health caregivers, and limited evidence-based information is available about thyroid cancer survivors’ long-term management needs, goals, and targets.
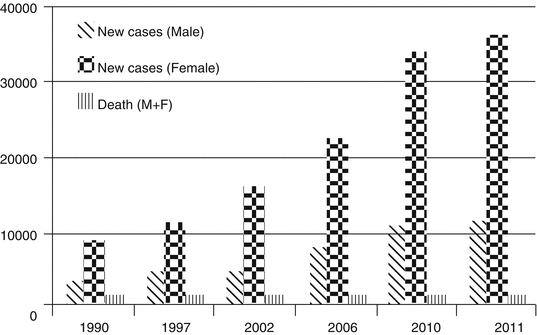

Fig. 14.1
New cases of thyroid cancer and thyroid cancer-related deaths that occurred each year in the United States between 1990 and 2011
Sequelae of Cancer Therapy in Thyroid Cancer Survivors
Thyroid cancer survivors are generally healthy individuals who lead a productive life after treatment. However, many survivors may face a wide range of long-lasting late effects of their disease in their near-normal lifespan. A report from MD Anderson showed that two-thirds of thyroid cancer survivors reported that their cancer or cancer treatment led to significant, lasting symptoms such as fatigue (Schultz et al. 2003). Similarly, reports from other institutions showed impaired quality of life in thyroid cancer survivors compared with control patients, independent of thyroid-stimulating hormone (TSH) levels (Tan et al. 2007; Hoftijzer et al. 2008).
Surgical Complications
Surgery is the most important initial treatment for thyroid carcinoma. Disease burden at the initial diagnosis and the presence of lymph node metastases determine the extent of initial surgical resection. Not surprisingly, more extensive surgery carries a higher risk for unwanted side effects, including vocal cord paralysis, regional pain, and hypocalcemia, among others. Fortunately, many of these complications tend to be transient, although some patients may experience long-lasting complications.
Hypoparathyroidism
Hypoparathyroidism is a known complication after total thyroidectomy. In most cases, patients develop transient hypocalcemia requiring short-term medical therapy. However, a small percentage of these patients may have permanent hypoparathyroidism, requiring frequent laboratory tests and medication adjustments as needed to maintain serum calcium levels in a narrow range to avoid problems from hypocalcemia, hypercalciuria, or hypercalcemia.
A growing number of surgical oncologists are performing central neck dissection with the initial total thyroidectomy in patients with differentiated thyroid carcinoma because of the high rate of lymph node metastasis in these patients. However, a higher incidence of transient and permanent hypoparathyroidism has been reported in patients who undergo central neck dissection than in patients who do not, and this should be considered when the patient is young and has a low risk of recurrence and a long life expectancy (Rosenbaum and McHenry 2009).
Implantation of parathyroid tissue can reduce both short-term hypocalcemia and permanent hypoparathyroidism after total thyroidectomy. The surgical expertise and extent of surgery are very important factors in determining the risk of surgical complications (hypoparathyroidism and vocal cord injury) in patients with thyroid cancer.
Vocal Cord Dysfunction
Preserving voice quality after thyroid surgery is an important goal for most patients. Transient recurrent laryngeal nerve dysfunction occurs in about 5–10% of all patients who undergo total thyroidectomy, and it usually improves spontaneously. Permanent recurrent laryngeal nerve injury is estimated to occur in about 1–2% of all patients who undergo total thyroidectomy. In some cases the injury may go unrecognized, but it can also lead to permanent hoarseness, coughing, and food aspiration. In a small number of patients with locally advanced thyroid cancer, those who underwent reconstruction of the recurrent laryngeal nerve at the time of thyroidectomy were found to have better vocal cord function than those who did not undergo this procedure (Hartl et al. 2005). In patients who develop unilateral recurrent laryngeal nerve damage, various procedures could be done to improve voice quality and reduce aspiration, including the medialization of the paralyzed vocal cord.
Limitation of Mobility
Limitation of neck-shoulder-arm mobility, local pain, and reduced sensation may occur after thyroid surgery, especially if wide neck dissection is also performed. In most cases, symptoms improve with conservative management and physical therapy. An estimated 5–15% of patients who undergo neck dissection are thought to be at risk for shoulder dysfunction, especially when the surgery is extended to the posterior neck triangle (level V). Upper extremity dysfunction can occur as a result of surgical injury, but it is usually mild; less than 10% of affected patients have reported severe impairment. Quality of life is also reduced in patients who develop neck pain and reduced shoulder mobility after surgery for head and neck malignancies (van Wilgen et al. 2004).
Radioactive Iodine Therapy Complications
Radioactive iodine (RAI) is often administered as an adjunct treatment in patients with differentiated thyroid carcinoma and is very well tolerated in most cases. However, concern is growing about unnecessary radiation exposure in patients with thyroid cancer who are at low risk for recurrence, who may not benefit from RAI therapy but could have a small risk for complications.
Oral and Ocular Complications
Because salivary and lacrimal glands have iodine uptake transporters, RAI uptake and accumulation occurs in these glands after RAI exposure, leading to problems such as salivary gland pain, xerostomia, altered taste, dental caries, salivary calculi, enlarged salivary glands, and excessive tearing secondary to lacrimal duct obstruction. A significant reduction in lacrimal gland secretion was observed in patients who had undergone RAI therapy, but similar reductions were also observed in patients who had not undergone the therapy (Fard-Esfahani et al. 2007). Furthermore, no correlation was found between RAI dose and tear production based on results of the Schirmer test. RAI therapy can also exacerbate coexisting Graves ophthalmopathy. Because some patients with differentiated thyroid carcinoma have Graves disease at the time of initial diagnosis, careful assessment is needed in these patients to avoid progression of exophthalmos.
Reduced salivary function in the first year after RAI therapy has been reported in 26–58% of patients with thyroid cancer who received the treatment, but only 5% had long-term side effects (Hyer et al. 2007; Grewal et al. 2009). The long-term adverse effects of RAI therapy on the salivary glands are most noticeable in the parotid glands, leading to a sense of dysphagia. Although conclusive evidence of benefit is lacking, sour candies are widely used to increase salivary flow at the time of RAI therapy with the hope of reducing salivary gland damage. However, emerging literature has suggested that this practice is potentially harmful, increasing salivary gland exposure to radiation by stimulating the salivary gland cells (Nakada et al. 2005).
Amifostine was proposed as a cytoprotective agent to reduce salivary gland damage after RAI therapy; however, a recent review article did not find conclusive evidence to support this claim (Ma et al. 2010), and the use of amifostine to protect salivary glands after RAI treatment remains questionable. The use of pilocarpine along with dexamethasone and intensive oral hygiene also was not found to reduce oral complications after RAI therapy (Silberstein 2008). Sialendoscopy has recently been used to treat selected patients who developed sialadenitis after RAI therapy. A retrospective review in 12 women with RAI-induced sialadenitis showed that mucous plugs and ductal stenosis were the most common abnormalities in these patients and that sialendoscopy use resulted in symptomatic improvement in 75% of patients (Bomeli et al. 2009).
Gonadal Effects
In male patients, testosterone levels have been shown to remain stable after RAI therapy. However, dose-dependent increases in follicle-stimulating hormone and luteinizing hormone levels have been observed in male patients during the first 6 months after RAI exposure, followed by spontaneous normalization after about 18 months, suggestive of RAI-related damage to the germinal epithelium. The radiation dose to the testicles has been found to depend on the RAI dose used, with an estimated median radiation dose of 6.4 cGy to each testicle in patients receiving 3 GBq (81 mCi) of RAI and 21.2 cGy to each testicle in patients receiving 9.2 GBq (249 mCi) of RAI (Hyer et al. 2002). Children born to men treated with RAI have not been found to have an increased risk for congenital malformations. Although the testicular exposure to radiation that occurs with RAI therapy is not enough to cause permanent damage to the testicles, one-third of male patients who underwent RAI therapy were found to experience reduced sperm count during follow-up (Esfahani et al. 2004). Patients concerned about fertility issues can request semen analysis and possibly opt for semen banking prior to RAI therapy.
In women treated with RAI, menopause was shown to occur at an earlier age compared with women with goiters who did not receive RAI (Ceccarelli et al. 2001). A minority of young women (younger than 40 years) who underwent RAI therapy were found to experience transient irregular menses without obvious harmful effects on fertility or pregnancy outcomes (Vini et al. 2002).
Secondary Malignancies
The risk of secondary malignancies after RAI therapy is less well established. However, in a review of a large cohort of patients with thyroid cancer, the risk of second primary malignancies in patients treated with RAI increased in a dose-dependent manner compared with the risk of second primary malignancies in the general population; an estimated extra three cases of leukemia were observed in every 10,000 patients receiving 100 mCi of RAI during 10 years of follow-up (Rubino et al. 2003). The risk of developing leukemia after RAI therapy is significantly increased, although the risk of developing solid tumors is less clear. Other studies have also shown an increased risk of developing leukemia and salivary gland malignancies after exposure to RAI but disputed the increased risk of developing other solid tumors (Sawka et al. 2009; Iyer et al. 2011). However, patients with thyroid cancer are subject to close clinical monitoring and surveillance, and this could lead to detection bias (i.e., more malignancies are found in this group compared with the general population).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree