83% papillary
60% papillary
23% follicular variant of papillary
10% follicular thyroid cancer
5% medullary
2% other
Papillary Thyroid Cancer
Pathologic features of papillary thyroid cancer include psammoma bodies and nuclear ground glass appearance, longitudinal grooves, and inclusions [32]. Histologic subtypes of papillary thyroid cancer seen in children include classic, solid, follicular, and diffuse sclerosing variants. The diffuse sclerosing variant is important to consider because it is more common in children, especially younger children, than in adults and it has a unique clinical presentation of enlargement of an entire thyroid lobe or the entire gland rather than as a nodule within the gland [36]. The relative prevalence of this variant has led to the recommendation that children presenting with diffusely enlarged thyroid glands or lobes have ultrasound imaging to evaluate for findings of malignancy [1].
Papillary thyroid cancer is often multifocal (30–60%) and bilateral (30%) and neck lymph node metastases are present in at least 30–60% of children at diagnosis [37–40]. Pulmonary metastases are also common (10–25%) in children at diagnosis, usually when neck lymph nodes metastases are present [41, 42]. Overall, at the time of diagnosis, children with papillary thyroid cancer are more likely than adults to have tumor extending beyond the capsule of the thyroid, regional lymph node metastases, and pulmonary metastases, even when controlled for tumor size and histology [37–39, 41, 42]. But despite having more advanced disease at the time they present, children with papillary thyroid cancer have an excellent prognosis.
Follicular Thyroid Cancer
Follicular thyroid cancer is relatively rare [5] and it is usually seen in adolescents rather than younger children. Unlike papillary cancer there is less of a female preponderance [41] and its incidence seems to be decreasing rather than increasing [43]. The risk factors for developing follicular thyroid cancer are different than the risk factors for developing papillary thyroid cancer with iodine deficiency related to follicular thyroid cancer but not papillary cancer [44, 45] and radiation exposure related to papillary thyroid cancer but not follicular cancer [37]. Follicular thyroid cancers are common thyroid tumors in the PTEN syndromes so patients with follicular thyroid cancers should be closely evaluated for PTEN syndromes [25].
Follicular neoplasms are typically encapsulated. The cytological features of follicular thyroid cancers and benign follicular adenomas are identical and it is only possible to make the diagnosis of malignancy when there is tumor invasion through the capsule or into blood vessels. This explains why FNA can only identify a follicular tumor and not a follicular malignancy. Histologic subtypes of follicular thyroid cancer include Hürthle cell, clear cell, and insular variants. These less differentiated variants are all rare in children [46] and tend to be associated with more aggressive disease and a worse prognosis in adults [47].
The clinical presentation and behavior of follicular thyroid cancer are distinct from the more common papillary thyroid cancer with fewer neck lymph metastases but rather a propensity for hematogenous spread and lung metastases. Follicular and papillary thyroid cancers have the same excellent prognosis [5].
Other Thyroid Cancers
Other, non-differentiated thyroid cancers in children include medullary and anaplastic thyroid cancer and lymphomas. Medullary thyroid cancer derives from parafollicular C cells that produce calcitonin rather than follicular cells that produce thyroid hormones. This tumor is reviewed in Chap. 31.
Anaplastic thyroid cancer accounts for <2% of thyroid cancers of all ages. It is mainly a disease of the elderly with the large majority of afflicted patients more than 60 years of age. Anaplastic thyroid cancer is very rare in children. Histologically, it is undifferentiated and similar to non-Hodgkin lymphoma, medullary thyroid carcinoma, the insular variant of follicular thyroid carcinoma, and poorly differentiated carcinoma that has metastasized to the thyroid. Modern immunohistochemistry techniques should make diagnostic confusion less likely. Anaplastic thyroid cancer is also functionally primitive and it does not concentrate iodine so radioactive iodine is not useful for treatment. Anaplastic thyroid cancer grows rapidly, invades locally, and often presents with symptoms such as dysphagia and hoarseness. More than 40% of patients have distant metastatic disease when they present. It is a deadly disease with more than 80% of patients dead within a year of diagnosis and most are dead within 6 months [48].
Lymphoma of the thyroid is rare in adults and children. It usually presents as a relatively rapidly enlarging neck mass in a patient with a history of Hashimoto’s thyroiditis. Pathologically, lymphomas of the thyroid are usually large B-cell lymphomas, but other types of lymphoma are possible. The diagnosis of lymphoma can be made by fine-needle aspiration biopsy by an experienced pathologist. Surgery is limited to the biopsy and treatment consists of chemotherapy and radiation depending upon the type of lymphoma [49].
Clinical Presentations of Thyroid Cancer
Thyroid cancer usually presents as a thyroid nodule or an enlarged thyroid gland that may be noticed by the patient or the patient’s family or is found on routine physical exam. Thyroid nodules may not be visible or palpable and only be discovered on imaging studies done for screening purposes or for other reasons. Significant thyroid nodules are usually evaluated by fine needle aspiration (FNA) that allows for risk stratification that defines a risk of malignancy. For more on the evaluation of thyroid nodules see Chap. 3.
Thyroid cancer may also be discovered in the surgical specimen after resection of part or all the thyroid gland done for diagnosis of a thyroid nodule or for a benign disease. Finally, thyroid cancer can present with a neck lymph node metastasis or even distant metastatic disease. As noted previously, children and especially younger children are more likely than adults to present with advanced disease [37, 41, 50].
Evaluation
The preoperative evaluation (or staging) of a patient with thyroid cancer usually begins during the evaluation of a patient’s thyroid nodule (see Chap. 3). A full history and physical examination should be done with directed questioning about symptoms of hoarseness, voice change, dysphagia, neck pain, and the onset and rapidity of growth of any neck mass. Any history of radiation exposure and family history of thyroid cancer or any malignancy should be noted. A full physical exam should be done with special attention to the thyroid gland and neck lymph nodes. If not already done, thyroid function tests should be obtained.
Critical for preoperative staging is a high-quality ultrasound of the thyroid gland and the entire neck with the goal of identifying cervical lymph node metastases that would dictate the performance of a neck dissection at the time of thyroid resection. If there is bulky lymphadenopathy or if there are any concerns about local invasion of the trachea, esophagus, or recurrent laryngeal nerve then more detailed imaging of the neck should be done with either a CT scan with contrast or MRI [1]. The potential disadvantage of a CT scan with (iodinated) contrast is that the iodine load given could delay postoperative staging and treatment with radioactive iodine. When abnormal lymph nodes are seen on imaging then FNA should be done to confirm the presence of metastases.
Nuclear medicine thyroid scans are not usually indicated preoperatively, except in the early evaluation of an undiagnosed thyroid nodule in a patient with a low TSH. If there is cervical lymph node metastases then a chest X-ray or a chest CT scan could be considered, although any lung metastases would probably be identified on the postoperative radioactive iodine scan.
Treatment
Children with thyroid cancer have been treated by a variety of methods over the last 50 years with very high survival rates, even though recurrent disease and persistent is relatively common. The goal of current treatment is to maintain the very low disease-specific mortality that has been has been obtained in the past while minimizing the complications and morbidity associated with treatment. The treatment of children with thyroid cancer is best done by an experienced, multidisciplinary team of medical and surgical specialists working in an environment with all the resources and personnel to care for children [1]. The team approach is essential in all phases of treatment because the evaluation, surgical treatment, medical treatment, and long-term follow-up are closely linked and interdependent.
In brief, the treatment of children with thyroid cancer begins surgery to remove all gross disease in the neck—usually total thyroidectomy [51] and compartmental excision of any involved cervical lymph nodes. Postoperative staging follows surgical resection. Patients found to have persistent local disease or distant metastatic disease and patients who are thought to be at high risk for recurrent disease are treated with radioactive iodine for [3, 4]. The goal of the combination of surgical resection and radioactive iodine is to eliminate all disease that can be detected by diagnostic whole-body radioactive iodine scanning and serum thyroglobulin.
Surgical Treatment
The operations done for treatment of thyroid cancer should be done by a surgeon experienced in thyroid surgery [1], although how these surgeons should be identified is a matter of ongoing debate. The initial operation for a known thyroid cancer is usually a total or near-total thyroidectomy (near-total thyroidectomy defined as preserving 1–2% of the thyroid gland that is adjacent to the recurrent laryngeal nerve or blood supply to the superior parathyroid gland). Total thyroidectomy is now favored over the previously commonly done thyroid lobectomy because of the high risk for bilateral disease (noted previously in Section “Papillary Thyroid Cancer”) and the higher risk of local recurrence and later surgical procedures when less than a total thyroidectomy is performed [40, 52, 53]. Total thyroidectomy also makes postoperative follow-up with diagnostic radioactive iodine scans and serum thyroglobulin easier and more reliable. Unilateral thyroid lobectomy is sometimes favored for very small tumors, especially when there is no evidence of disease outside the thyroid gland and future radioactive iodine imaging and treatment is not likely. The anatomic principles of total thyroidectomy are discussed in detail in Chap. 1.
The most serious complications after total thyroidectomy include hypoparathyroidism and recurrent laryngeal nerve injury. Postoperative hypoparathyroidism with resulting hypocalcemia is caused by resection or devascularization of the parathyroid glands [54]. If devascularization is recognized then the parathyroid gland can be autotransplanted into the sternocleidomastoid muscle [55]. Transient hypocalcemia requiring postoperative treatment with calcitriol (the active form of vitamin D) and calcium is a relatively common problem after total thyroidectomy that can prolong hospitalization while the serum calcium levels stabilize. There are different strategies to deal with this problem including intraoperative monitoring of the parathyroid hormone (PTH) [56] and initiating early, prophylactic treatment of all patients with calcitriol and calcium [57]. Efforts to prevent recurrent laryngeal nerve injury have been concentrated on methods of nerve monitoring which although technically possible have not been found to reliably decrease the risk of nerve injury [58].
Surgery for follicular neoplasms usually consists of an initial thyroid lobectomy. Frozen section may be useful to look for unexpected papillary thyroid cancer but will not be able to differentiate a benign from a malignant follicular tumor. When the follicular neoplasm is benign then no further surgery is needed. When the follicular tumor is malignant then careful consultation with pathology, endocrinology, oncology, and nuclear medicine experts is needed to decide if completion thyroidectomy is needed or if lobectomy will be sufficient resection. Smaller tumors that have minimal evidence of invasion may potentially be treated by lobectomy alone while larger tumors and those that are more invasive histologically are probably best treated by completion thyroidectomy. The usual lack of neck lymph metastases means that neck lymph node dissections are typically not part of surgical treatment of follicular cancer.
The frequent spread of papillary thyroid cancer to neck lymph nodes and the treatment goal of removing gross disease lead to the frequent performance of lymph node resections [40]. These procedures can be therapeutic, prophylactic, unilateral, or bilateral. It is optimal that any lymph node resection be done at the time of the thyroid operation, which is why preoperative assessment of the neck lymph nodes is so important.
When lymph nodes are removed it should be done as “compartment dissection” rather than as “berry-picking”, the removal of only obvious, enlarged lymph node [59]. The definition and reporting terminology of neck dissections for thyroid cancer have been standardized [60, 61]. The central neck or anterior compartment is immediately adjacent to the thyroid gland and is designated level VI. Level I is also central but higher in the neck in the submental area (level IA) and submandibular area (level IB). Levels in the lateral neck are II through V. Levels IIA and IIB are the upper jugular group with IIA being anterior (medial) and IIB being posterior (lateral to the spinal accessory nerve). Level III is the middle jugular area from the hyoid bone superiorly to the cricoid cartilage inferiorly. Level IV is the lower jugular area from the cricoid cartilage to the clavicle and Level V is the posterior triangle area (Fig. 4.1).
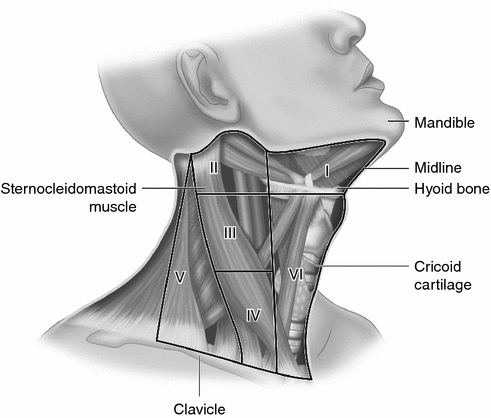

Fig. 4.1
Neck lymph node levels (see text for descriptions)
Papillary thyroid cancer most commonly metastasizes to the central area, level VI, on the same side as the tumor. When there is preoperative evidence of abnormal lymph nodes in the central neck (or in the lateral neck) then central neck dissection is recommended [1, 60]. Unfortunately, neck ultrasound is less reliable at identifying abnormal lymph nodes in the central neck than in the lateral neck [62]. Also, it is not clear if ultrasound size criteria of the tumor or the lymph node that have been used for adults to identify patients at high risk for metastatic disease are applicable to children [63]. Central neck lymph node dissections increase the risk of recurrent laryngeal nerve injury and permanent hypoparathyroidism so there is considerable debate on when and how (unilateral or bilateral) they should be performed [64, 65].
Lateral neck dissections are those compartmental lymph node resections beyond the central compartment (level VI). Lateral neck dissections are recommended when there is preoperative evidence of lymph node metastases in those compartments to decrease the chance of persistent and recurrent disease [37, 40]. The dissection can be tailored based on the preoperative staging but often involves levels III, IV, V, and II [66].
Although thyroid surgery in adults is increasingly being done on an outpatient basis, most children, especially younger children and those undergoing a total thyroidectomy with or without neck dissection are admitted after their operation. They are observed for potential bleeding, airway compromise, recurrent laryngeal nerve injury, and hypocalcemia due to hypoparathyroidism. Operative complications are directly related to younger ages and the extent of the operation with recurrent laryngeal nerve injury reported in 3.8% of children 6 years and under, in 1.1% of children 7–12 years, and in 0.6 of patients 13–17 years of age [67]. TSH usually stimulates differentiated thyroid cancer so after surgery thyroid hormone replacement is given to suppress TSH with the target TSH level being lower in patients at higher risk [1].
Postoperative Staging
After the initial operation postop evaluation (staging) is done to identify patients who would benefit from postoperative radioactive iodine treatment and to help define the intensity of follow-up care needed. This staging is dependent upon the surgical pathology, a postoperative diagnostic whole-body radioactive iodine scan, and serum thyroglobulin level [1]. Staging is best done within 12 weeks of surgery.
Pathologic staging for children is a modification of the adult method by the American Thyroid Association that is designed to better risk stratify patients [1]. There are three risk groups—Low, Intermediate, and High [1]. The Low risk group has either no neck lymph node metastases or only microscopic metastases to a small number of central (level VI) lymph nodes. Low-risk patients have a low risk of distant metastases but some risk of recurrent or persistent disease in the neck lymph nodes. Intermediate risk patients have extensive central neck lymph node metastases or minimal lateral compartment neck lymph node metastases. They are also at a low risk for distant metastases but have an increased risk (compared to Low risk patients) of recurrent or persistent disease in the neck lymph nodes. High-risk patients have locally invasive disease, extensive metastases to lateral compartment lymph nodes, or distant metastases. They have the highest risk of recurrent or persistent regional or distant disease [1].
The staging for Low-risk patients consists of serum thyroglobulin levels while they are euthyroid; i.e., when their TSH is suppressed by their thyroid hormone replacement. Thyroglobulin is a glycoprotein made by follicular cells and follicular cell malignancies and its presence correlates well with the presence and magnitude of metastatic disease in patients who have had their normal thyroid removed [68]. This value can then be followed in the future to observe trends. If the thyroglobulin is initially low and increases over time then it would indicate recurrent disease.
Staging for patients who are Intermediate and High Risk consists of serum thyroglobulin levels when their TSH levels are elevated (the TSH will tend to stimulate any persistent disease) and diagnostic whole-body radioactive iodine (RAI) scans, although some Intermediate risk patients may not need diagnostic scanning [1]. Diagnostic radioactive iodine scans are done with either I-123, which is ideal but more expensive, or I-131, which is the same isotope, used for treatment.
Postoperative Radioactive Iodine Treatment
Radioactive iodine treatment is given to treat known residual or metastatic disease and to prevent recurrent disease [69]. Enthusiasm for its routine use has been tempered by the observation that when children with differentiated thyroid cancer are followed for a very long time that those treated with radioactive iodine have an increase in all-cause mortality compared to those not treated with radioactive iodine. This increased mortality is mainly due to an increased risk second malignancy in those treated with radiation [52, 70].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree