Invasive cancer
Invasive + In situ cancer
Incidencea
Thyroid
Incidencea
Thyroid
All sites
Thyroid
%
All sites
Thyroid
%
All ages
Male and female
466.4
11.0
2.4 %
522.5
11.0
2.1 %
Female
413.4
16.3
3.9 %
482.3
16.3
3.4 %
Male
542.5
5.6
1.0 %
585.9
5.6
1.0 %
Age 15–39
Male and female
68.0
8.7
12.7 %
80.5
8.7
10.8 %
Female
83.4
14.5
17.4 %
101.4
14.5
14.3 %
Male
52.7
3.0
5.6 %
59.8
3.0
4.9 %
Pediatric thyroid malignancies arise typically from one of two normal thyroid cell populations, either the thyroid follicular epithelium or the parafollicular C cell, which has a distinct embryologic origin. Differentiated thyroid carcinoma (DTC) – including papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and their variants – arises from the former, whereas medullary thyroid carcinoma (MTC) arises from the latter. Appreciating this major histologic distinction is fundamental to understanding the differences in the biologic behavior and treatment applicable to these very different thyroid cancers. While the majority of PTC subtypes are represented by low-risk ones like the classic, the solid/trabecular, microcarcinoma, diffuse sclerosing, follicular, and encapsulated follicular [4, 5], rare cases also belong to high-risk PTC according to adult prognosis. As an example, we have recently described three patients with tall cell variant that has in adult a fourfold risk of relapse and twofold relapse-related risk of death. These three patients, on the contrary, despite hemithyroidectomy in two of them, were alive without relapse after almost 30 years thus showing the peculiar good outcome of childhood and adolescence DTC despite any other adult-customized feature [6].
During the last decades, changes in clinical presentation have been found in children: specifically, palpable cervical adenopathy, once a common presenting symptom, has decreased from 63 to 36 %; invasion of contiguous structures has dropped from 31 to 6 %, and distant metastases has fallen from 19 to 6 %; on the other hand, presentation as a solitary thyroid nodule has increased from 37 to 73 % among children [7]. Although poorly differentiated and frankly anaplastic thyroid carcinomas can occur in the adolescent and young adult population, they are exceedingly rare. Therefore, the current chapter will focus only on DTC and MTC.
9.2 Epidemiology, Survival, and Overdiagnosis
9.2.1 Incidence During 1975–2011
The incidence of thyroid cancer among 15- to 39-year-olds in the USA has increased steadily since 1990, especially in females in whom the thyroid cancer occurred in one in every five females by 2010 (Fig. 9.1).
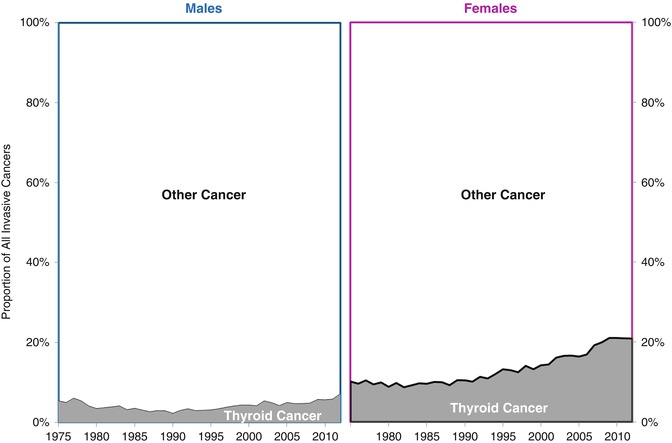

Fig. 9.1
Proportion of all invasive cancers that is thyroid cancer in AYAs of age 15–39, by year, 1975–2011, SEER9
9.2.2 Incidence During 2000–2011
During 2000–2011, the incidence of thyroid cancer was distinctly more common in females, at all ages. The peak incidence occurred at an older age in males, between 65 and 75 years, than in females, between 50 and 55 years of age. In AYAs (Fig. 9.2), the incidence increased eightfold between 15 and 40 years of age in females and ~5-fold in males. AYAs had the highest ratio incidence in females to male, ranging from 4.3 to 6.4 by 5-year age subgroup and peaking between 20 and 25 years of age.
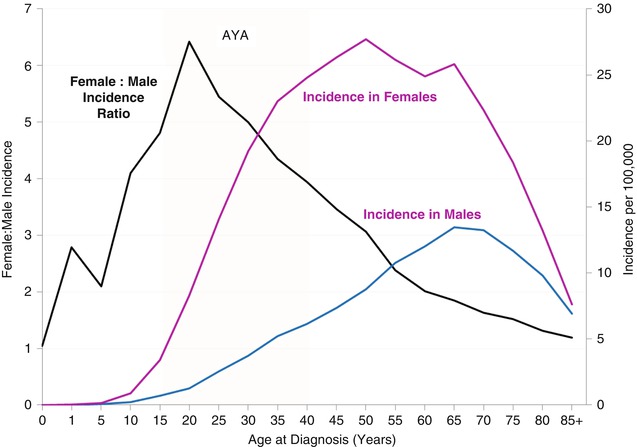

Fig. 9.2
Thyroid cancer female/male incidence ratio and incidence by age and sex, SEER18, 2000–2011
Metastatic cancer at diagnosis of thyroid cancer was uncommon before age 50 and didn’t account for more than 5 % of the cases until age 70 (Fig. 9.3). In AYAs (Fig. 9.3, yellow highlight), regional + distant stage accounted for 40–50 % of all cases in patients <30 years of age, and localized presentation accounted for 45–65 %. Males had proportionately more regional and distant disease at diagnosis up to the age of 75 than females. In AYA males (Fig. 9.3), the incidences of localized and regional disease at diagnoses were similar, whereas in women, localized disease predominated from the age of 20 and up.
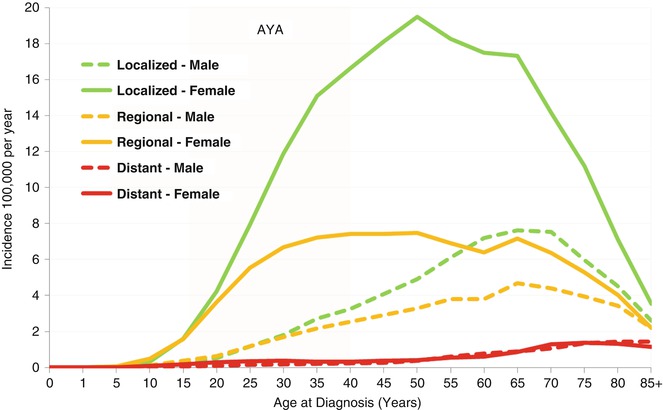

Fig. 9.3
Thyroid cancer incidence by age, sex, and extent of disease at diagnosis, SEER18, 2000–2011
In both females and males, non-Hispanic whites had the highest incidence of thyroid cancer, and blacks and native North Americans had the lowest (Fig. 9.4). Hispanic AYA females had a higher relative incidence than other major races/ethnicities than in Hispanic AYA males, with the former more similar to non-Hispanic whites and Asians/Pacific Islanders and the latter similar to blacks and native North Americans.
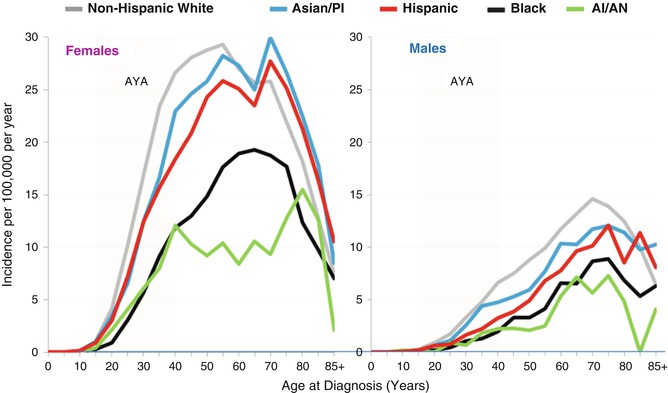

Fig. 9.4
Thyroid cancer incidence by age and race/ethnicity and sex, SEER18, 2000–2011. PI, Pacific Islander; AI/AN, American Indian/Alaska Naïve (native North Americans)
9.2.3 Incidence Trends During 1976–2011
The incidence of thyroid cancer increased in females and males and primarily in AYAs (Fig. 9.5). In AYA females, the increase began in the early 1990s and was greater than in AYA males. In AYA males, the increase was more recent, primarily since 2000.
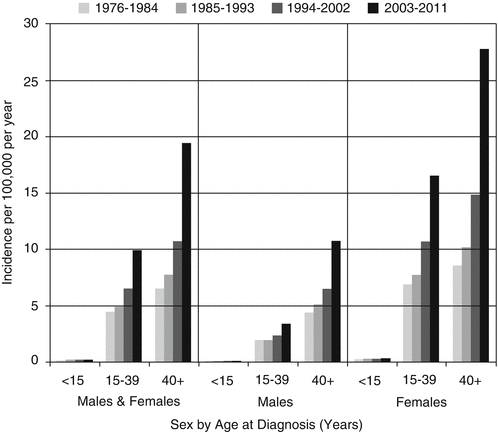

Fig. 9.5
Thyroid cancer incidence by age, race/ethnicity, and sex, SEER18, 2000–2011
Whereas there was no consistent change in incidence of AYAs with distant disease at diagnosis, the incidence of regional and localized disease increased, with localized disease accounting quantitatively for most of the overall increase (Fig. 9.6).
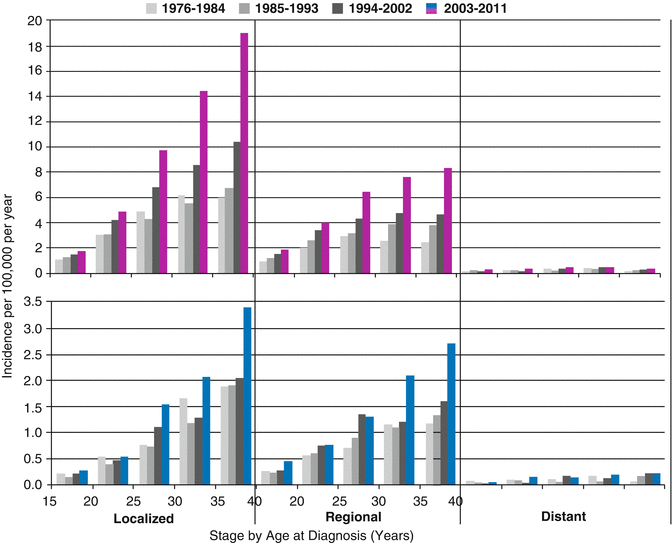

Fig. 9.6
Thyroid cancer incidence by age, race/ethnicity, sex, and extent of disease at diagnosis, SEER18, 2000–2011
9.2.4 Survival During 2000–2011
Including those with distant metastases at diagnosis, AYAs had the best 5-year thyroid cancer-specific survival, 99.9 % for localized disease, 99.8 % for regional disease, and 97.7 % for distant disease. In AYAs <30 years of age at diagnosis, the 5-year thyroid cancer-specific survival was >98.6 % for those with distant metastases at diagnosis. For AYAs of all ages, the rate was >99.8 % for regional and localized disease. In AYAs with distant metastases at diagnosis, males had a worse 5-year thyroid cancer-specific survival than did females, with the worse outcome, 92 % in males 35-29 years of age. Overall all stages, male and female AYAs had the same survival rate. Not until age 65 did males have a worse outcome, albeit by just a few percent (Fig. 9.7).
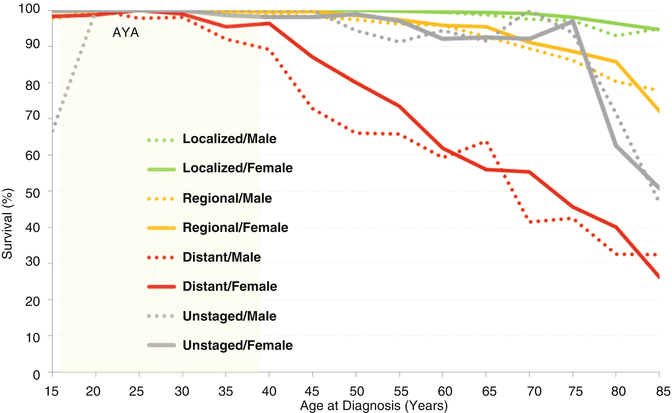

Fig. 9.7
Thyroid cancer survival by age, sex and extent of disease at diagnosis, SEER18, 2000–2011
9.2.5 Survival Trends During 1976–2011
The 5-year thyroid cancer-specific survival has been near or at 100 % in AYAs since 1976. Only in patients over 40 years of age was the survival significantly <100 %, and it improved during 1976–2010, from 89 to 96 % in males and from 91 to 98 % in females (Fig. 9.8).
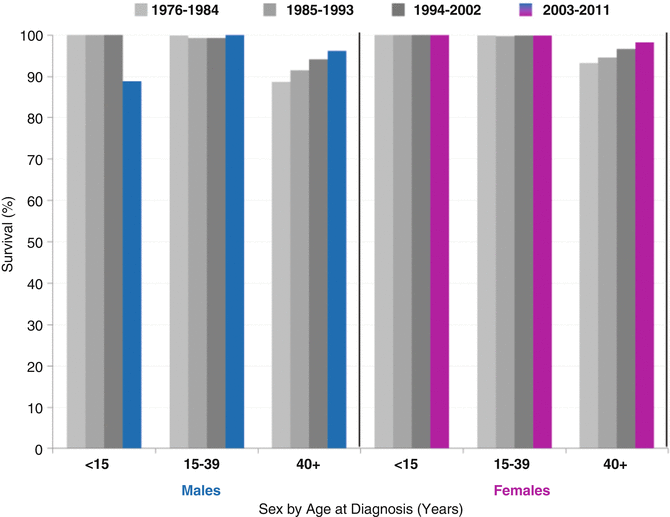

Fig. 9.8
Five-year thyroid-specific cancer survival trends by age and sex, 1976–2011, by 9-calendar year intervals, SEER18
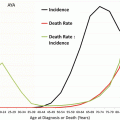
9.2.6 Death Rates and Mortality Trends During 2000–2011 and Comparison with Incidence Trends
The thyroid cancer death rate was miniscule compared with the incidence rate up to the age of 55 (Fig. 9.9). In AYAs, the death rate (Fig. 9.9, inset) was 0.7–0.8 % of the incidence rate (Fig. 9.9, main chart) in 20–34-year-olds and 1.3 % in 35–39-year-olds. Among AYA females, one thyroid cancer death was recorded for each 560 new cases; in AYA males, one death occurred for each 123 new cases.
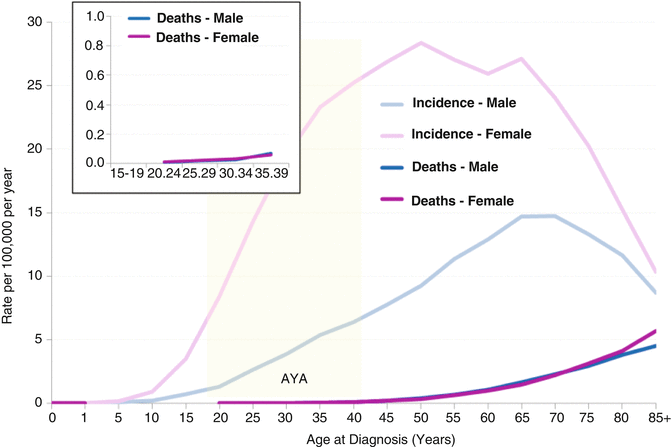
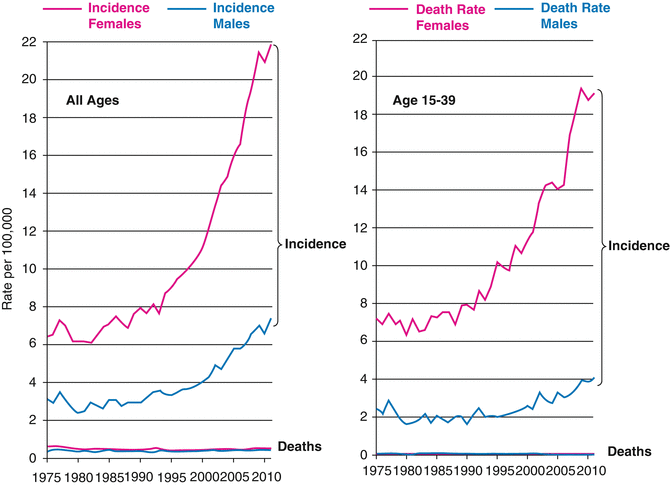

Fig. 9.9
Thyroid cancer incidence and death rates by sex and age, 2000–2011

Fig. 9.10
Annual incidence of and deaths from thyroid cancer since 1975 in SEER regions, all ages (left panel) and AYAs (right panel)
The striking increase in new cases of “thyroid cancer” since 1990 has not affected the mortality rate of thyroid cancer, either over all ages (left panel) or in AYAs specifically (right panel). The disassociation is more dramatic in females than males. Of three major explanations, (1) the expected increase in the death rate has not yet occurred and will do so in the future, (2) current therapy is completely curative, and (3) the excess cases are overdiagnosed (nonlife threatening, not “cancer”), and therapy is not necessary, the latter is most likely.
The striking increase in new cases of “thyroid cancer” since 1990 did not affect the incidence of metastatic disease at diagnosis, in either females (Fig. 9.11, left panel) or males (Fig. 9.11, right panel), which is most likely due to overdiagnosis. This “epidemic” has not included regional disease which indicates that more therapy, either more surgery, node dissections, and/or radiotherapy, was administered unnecessarily to those men and women who were overdiagnosed.
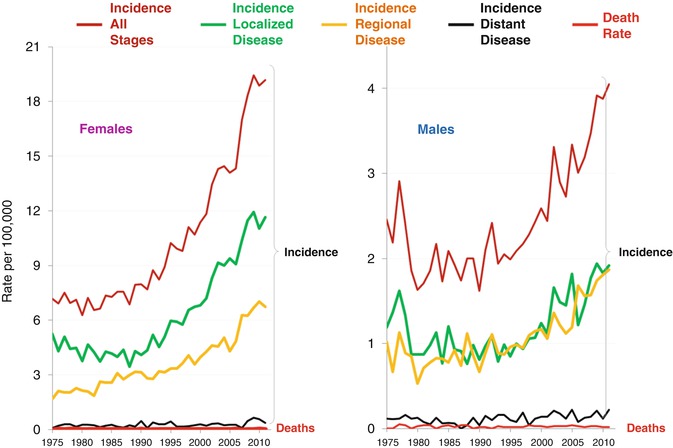

Fig. 9.11
Annual incidence of thyroid cancer overall and by stage and of thyroid cancer deaths among 15–39-year-olds since 1975 in SEER regions by sex
Of all the cancers in AYAs, thyroid cancer has had the greatest increase in incidence relative to the change in mortality rate (Fig. 9.12). During the past decade, AYAs have had greater increase in the incidence of thyroid than any other cancer except kidney cancer and average of 5 % per year or nearly 50 % during the decade, whereas deaths from thyroid cancer in the age group did not substantively change. The difference between the average annual percent changes (AAPCs) among 15–39-year-olds in comparison with the AAPC of the death rate was greater for thyroid cancer than any other cancer.
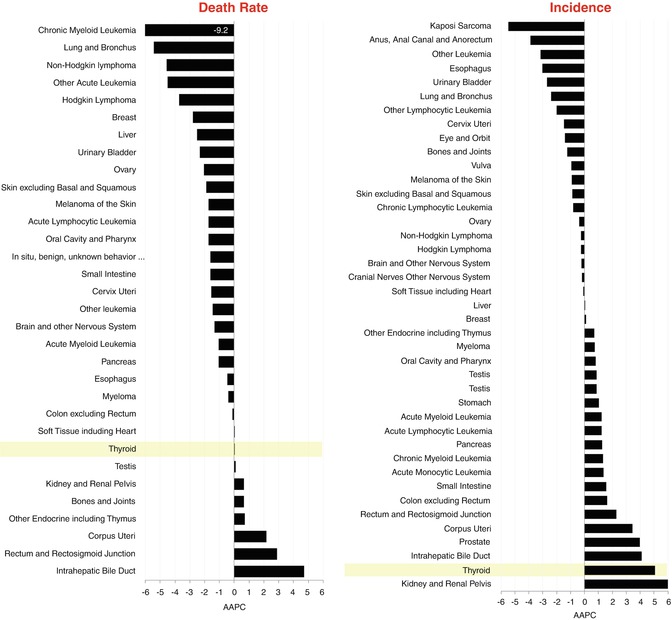

Fig. 9.12
Average annual % change (AAPC) in age-adjusted death rate (left panel) and incidence (right panel) in 15- to 39-year-olds by type of cancer, 2000–2011, USA
9.2.7 Overdiagnosis
Overdiagnosis of thyroid cancer is more problematic for AYAs than for either younger or older person and more than any other cancer. The problem has been observed in the USA [8], Canada [9], Australia [10], and South Korea [11]. The increase in incidence of thyroid cancer and lack of change in thyroid cancer mortality in Figs. 9.10 and 9.11 suggest that two of every three AYAs with thyroid cancer in the USA are being overdiagnosed, the highest rate of overdiagnosis of any cancer. The cause has been attributed to the increased availability and use of more sensitive imaging techniques such as ultrasound, CT scanning, and MRI scanning [12] that detect subclinical nodules and that appear to pathologists as cancer but that do not affect the patient in her or his lifetime. Virtually all persons diagnosed with thyroid cancer are treated: roughly two thirds undergo radical thyroidectomy, and one third undergo subtotal thyroidectomy. The tumors being excised are getting smaller – at one center, the proportion of patients undergoing surgery for a tumor measuring less than 1 cm in diameter increased from 14 % in 1995 to 56 % 10 years later [11]. Despite guidelines in South Korea recommending against evaluation and surgery for tumors less than 0.5 cm in diameter, one quarter of surgical patients now have tumors that fall into this category. Thyroid cancer surgery has substantial consequences for patients. Most must receive lifelong thyroid replacement therapy, and a few have complications from the procedure. An analysis of insurance claims for more than 15,000 Koreans who underwent surgery showed that 11 % had hypoparathyroidism and 2 % had vocal cord paralysis [11]. As of 2015, there is still little to no evidence that this extraordinary problem of a high rate of unnecessary therapy and associated compromise of health-related quality life has been addressed.
In the USA, the evolution of overdiagnosis of thyroid cancer accounts for a substantial proportion of the increase in incidence of invasive cancer in AYA females. When thyroid cancer is excluded, the statistically significant increase in incidence during 2000–2012 is eliminated (Fig. 9.13).
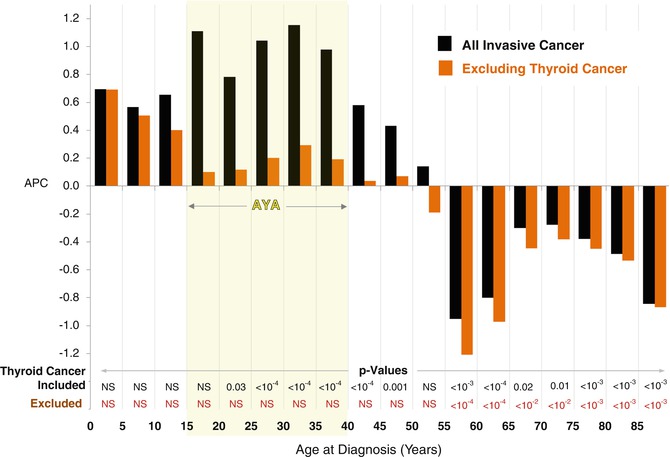

Fig. 9.13
Annual % change (APC) in incidence of invasive cancer in females by age with and without thyroid cancer, SEER18, 2000–2012
Also, the inclusion of such cases artificially increases the survival rate of a cohort because the cases per se cause no deaths. Among females, who have a much higher proportion of thyroid cancer than males, one third (32 %) of the improvement in survival of AYAs with cancer during 2000–2008 was due to thyroid cancer and its overdiagnosis (Fig. 9.14).
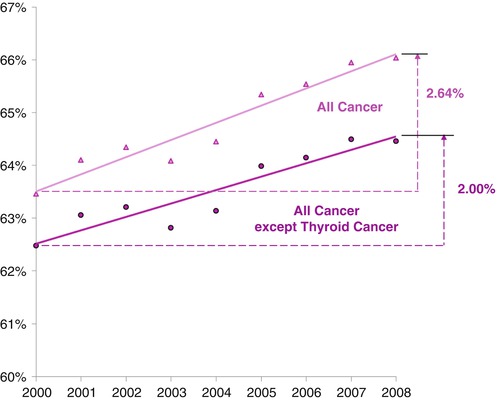

Fig. 9.14
Five-year relative survival of females with cancer, age of 15–39 years, by year of diagnosis, 2000–2008, with and without thyroid cancer, SEER
9.3 Differentiated Thyroid Carcinoma
9.3.1 Etiology/Pathology
Differentiated thyroid carcinoma (DTC) is the most commonly encountered thyroid cancer in childhood, with PTC representing about 80 % and FTC being roughly 20 % of malignancies that arise from the follicular epithelium [13–15]. The diagnosis of PTC and FTC is based upon unique histopathological features, and there are subtypes of each, including follicular cell, tall cell, diffuse sclerosing, columnar cell, and encapsulated variants in PTC. Variants of FTC include Hürthle cell (oncocytic), clear cell, and insular carcinoma. Certain tumor subtypes, such as the follicular and diffuse sclerosing variants of PTC, are more common in children and young adults as compared to older individuals [16]. Furthermore, as compared to the classical type found in older individuals, childhood PTC, particularly in patients less than 10 years of age, (1) may be unencapsulated and widely invasive throughout the gland and (2) may have a follicular and solid architecture with unique nuclear features and abundant psammoma bodies [17].
Despite the fact that PTC and FTC are both derived from the follicular epithelium and are treated in a similar fashion, there are some key differences in clinical behavior, specifically the risk and pattern of metastases. PTC is more likely to metastasize through lymphatic channels to regional neck lymph nodes. Hematogenous metastases, primarily to the lung, occur less frequently and typically only when locally metastatic disease is also present. FTC, on the other hand, is more prone to hematogenous metastases (affecting predominantly the lungs and bones); they metastasize less often to regional lymph nodes. Furthermore, PTC is more likely to be multifocal and bilateral [7]; FTC, in contrast, is usually a unifocal tumor.
It is a well-known phenomenon that the outcome of pediatric PCT is independent of strong prognostic factors of adults, such as low- versus high-risk histological subtype, extrathyroid local invasion into soft tissue of the neck, presence of distant metastases, site of distant metastatic spread, occurrence of relapse, and type of surgery [4, 18]. Interestingly, a review of 120 papillary carcinomas, in patients younger than 20 years, has evaluated in a multivariate analysis risk factors for disease-free-survival and has revealed that initial nodal manifestation was the most significant risk factor. The amount of thyroid excision and the use of radioiodine therapy did not correlate to the final outcome [19].
The major established environmental risk factor for the development of benign and malignant thyroid neoplasms, particularly PTC, is radiation exposure to the head and neck [20, 21]. Children, particularly those less than age 5 years, are much more sensitive to the tumorigenic effects of irradiation [21, 22]; this may in part be due to the higher rate of thyroid cell replication in children as compared to adults [16, 23, 24]. Since children are no longer treated with radiation for benign conditions, such as thymic enlargement, tonsillar hypertrophy, or acne, there are now fewer thyroid cancer patients with this well-established risk factor; however, the use of external beam radiotherapy to treat malignancies (especially Hodgkin’s disease) remains a significant risk for the development of thyroid carcinoma, even for many years after therapy is complete [25]. A specific paragraph has been dedicated to this subject. Although there are some conflicting data, it appears that cases of radiation-induced thyroid carcinoma are not significantly different in clinical behavior as compared to sporadic nonradiation-induced tumors [25, 26].
Internal ionizing radiation, such as that which occurred with the large environmental exposure to radioactive iodine from the Chernobyl nuclear accident, is another well-documented risk for the development of PTC, particularly in children less than 10 years of age at the time of exposure [27, 28]. Recent evidence suggests that the thyroid gland in younger children is better equipped to transport iodine as compared to older children [24]. Assuming that the mean radiation exposure per gram of thyroid tissue is inversely related to the age of the individual at exposure, it would make sense why the youngest children are most at risk for developing PTC after accidents such as Chernobyl. With an increased use of computed tomography scans in pediatric patients, there is an estimated risk of malignancy as high as one fatal cancer per 1,000 CT scans performed on children [29]
Researchers are beginning to unravel the molecular and genetic basis of the differentiated thyroid carcinomas. One of the major early somatic events that is associated with the development of papillary thyroid carcinoma is a chromosomal rearrangement linking the promoter region of an unrelated gene(s) (named PTC) to the carboxyl terminus of the RET (rearranged during transfection) proto-oncogene [16, 23, 28]. This occurs either because of a simple inversion of a segment of chromosome 10 (where RET resides) or a translocation of RET to a different chromosome. The RET/PTC rearrangement produces a chimeric oncogene, resulting in a constitutively activated form of the RET receptor tyrosine kinase (i.e., activation in the absence of ligand), thereby promoting tumorigenesis. Although it is believed that RET/PTC rearrangements may be critical for the development of pediatric- and radiation-induced PTC [30–36], some recent reports have challenged these conclusions [37].
Other important genes and gene products implicated in thyroid tumorigenesis and biological behavior include RAS and BRAF (important for intracellular signaling pathways; BRAF is implicated in PTC only), rearrangement of the TRK proto-oncogene (akin to RET but found in only a minority of PTCs), MET overexpression (mostly in PTCs), the p53 tumor suppressor gene (specifically involved in anaplastic thyroid cancer), and Pax8PPARγ1 translocations (follicular adenomas and follicular thyroid carcinomas only) [16, 23, 38, 39].
Approximately 3–5 % of patients with PTC have a family history of the disease [16, 40]. Having a positive family history may portend a worse prognosis, given that these cases appear to have more aggressive disease and shorter disease-free intervals after initial treatment [40, 41]. As of yet, the genetic basis for dominantly inherited non-MTC has not been elucidated. Other familial tumor syndromes in which there is an increased risk of DTC include familial adenomatous polyposis (Gardner syndrome), Cowden disease, and the Carney complex [16].
9.3.2 Diagnosis and Clinical Presentation
In childhood, DTC usually presents as an asymptomatic neck mass [42, 43]. Occasionally, the diagnosis may be made incidentally after the discovery of pulmonary nodules on a chest radiograph. In any individual younger than 20 years of age presenting with a solitary thyroid nodule, there is a higher likelihood of malignancy [14, 44]. The overall prevalence of thyroid carcinoma is about 20–25 % of thyroid nodules in children, compared to 5 % in adults [14, 16, 44, 45]. Symptomatic thyroid cancers (i.e., those associated with hoarseness, dysphagia, or cough, thus suggesting more locally advanced disease) are rare in young individuals. Uncommonly, thyroid carcinoma arises ectopically in a thyroglossal duct remnant or cyst. Arguably, this would be an unusual presentation of childhood thyroid carcinoma, but it must be kept in mind for patients presenting with a midline mass in the region of the hyoid. Finally, although most patients are euthyroid at the time of diagnosis, rare cases of differentiated follicular thyroid carcinomas can present as a functioning nodule associated with a suppressed thyroid-stimulating hormone (TSH) or frank thyrotoxicosis.
In children and young adults, it is not unusual for thyroid carcinoma to present only with cervical lymph adenopathy, and locally metastatic disease is indeed present at diagnosis in the majority of pediatric PTC cases [43, 47, 48]. In addition, children more often have disseminated disease at diagnosis, with lung metastases identified in up to 20 % of cases [17, 47, 49]. Metastases to other sites, such as the bone and brain, are rare. In the presence of a thyroid or laterocervical nodule in the neck of a child or an adolescent, the first diagnostic step is a thorough clinical neck examination, with clinical assessment of the site of the nodule (thyroid vs. node vs. others) and its characteristics (site, size, consistency and mobility), and direct or indirect evaluation of laryngeal or esophageal involvement by the neoplasm through the evaluation of their functional alteration (dysphonia and dysphagia). In a patient presenting with a painless thyroid nodule, the first subsequent procedure should be a high-quality neck ultrasound (US; together with fine-needle aspiration and biopsy, FNAB), which assists greatly with surgical planning [50]. In young patients, FNAB has not been utilized extensively, and the scanty studies reported are often in disagreement. Nevertheless, a meta-analysis certified FNA as a sensitive diagnostic test and a useful tool in diagnosing malignancy in pediatric thyroid [51]. The procedure should only be performed by experienced physicians and cytologists. US is useful in determining the size and appearance of the lesion, assessing for other nodules, ensuring the accuracy of FNA, and looking for evidence of metastatic lymphadenopathy. For these reasons, US should be considered even when the diagnosis of thyroid carcinoma is already known. Ultrasound characteristics suggestive of malignancy include indistinct margins, microcalcifications, and variable echotexture [52–54]. However, it should not be understated that the utility of ultrasound is greatly dependent upon the expertise of the ultrasonographer, particularly when it comes to identifying metastatic lymphadenopathy.
There remains some controversy about the definitive management of thyroid nodules in children. For example, biopsy (often using US guidance) is the recommended initial procedure in adults and can easily be accomplished in mature adolescents and young adults [46, 52, 55]. Although FNAB can also be easily performed in younger children, conscious sedation may be required. On the other hand, many experts feel that the initial diagnostic step should be surgery (i.e., lobectomy and isthmusectomy), given the higher likelihood that a thyroid nodule in a child, particularly when accompanied by palpable lymphadenopathy, is a carcinoma. Although this is a reasonable approach, it is our feeling that a preoperative FNAB (and subsequent pathologic diagnosis) allows for better operative planning and minimizes the need for a second surgery, particularly in children who present with a single thyroid nodule only.
Baseline thyroid function tests should also be obtained at presentation. Nuclear imaging studies using radioactive iodine or technetium pertechnetate are not very useful in the initial evaluation of these patients, except in those with a low TSH, because even benign thyroid nodules will be “cold” on nuclear imaging. In DTC, tumor cells typically retain the ability to produce the thyroid-specific glycoprotein, thyroglobulin (TG). Measuring TG is not routinely recommended in the initial evaluation of a thyroid neoplasm, because elevated TG levels are identified in a variety of benign thyroid processes, thereby lowering the specificity of this diagnostic test. Once a diagnosis of thyroid carcinoma is established, however, a baseline TG may be useful for follow-up. After a clinicopathological diagnosis of carcinoma, in the radical therapeutic approach, that follows conventionally the adult care with total thyroidectomy, lymphadenectomy, and radioiodine treatment of thyroid remnants, a “microstaging” (aimed at the detection of the subclinical neoplasm, for instance, by CT or MRI) is performed. A “macrostaging” (aimed at the detection of the clinically evident neoplastic burden) instead is considered adequate in the conservative approach that will be later described, considering as relevant only the grossly evident diffusion of the tumor [56].
9.3.3 Management
The initial care of adults with DTC is guided by consensus guidelines that can help the practitioner manage these patients [55]. However, it cannot be emphasized enough that established recommendations always need to be individualized for each patient, especially when dealing with children, adolescents, and young adults. It is imperative to note that no prospective clinical trials with randomized questions have been undertaken in children to determine the optimal therapeutic approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree