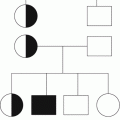
Primary features
Pathophysiology
Complications
Treatment
DIC
Abnormal coagulation (increased PT, APTT, reduced fibrinogen and platelets)
Underlying precipitant, e.g. placental abruption, sepsis
Excessive bleeding or thrombosis
Treat the underlying cause and support with blood components/products as required
PET
Hypertension (>140/90 mmHg), proteinuria (>0.3 g/24 h), edema
Dysfunctional spiral artery development and release of trophoblast derived factors, e.g. increased VEGF-1 (s-Flt-1) and sEng; reduced VEGF and PIGF
Thrombocytopenia, HELLP syndrome; renal impairment; eclampsia (convulsions); stroke; placental abruption; IUGR and preterm delivery
Symptomatic control of hypertension. If severe, delivery. Antiplatelets +/− LMWH for prevention in high risk women
HELLP
Upper abdominal pain and tenderness, nausea, vomiting, malaise, headache, rarely jaundice
Result of endothelial cell injury. Bilirubin is typically not raised, transferases are increased from only marginally to 20-fold. The degree of thrombocytopenia defines type (I, II or III)
DIC, placental abruption, acute renal failure, pulmonary edema, hepatic failure (occasionally requiring liver transplantation), hepatic rupture
Symptomatic control of seizures, hypertension and thrombocytopenia. If severe, delivery. PEX may be required. Steroids may improve thrombocytopenia
TTP
Primarily neurological and cardiac features acutely, but features in pregnancy may be non-specific
ADAMTS 13 deficiency. Either congenital TTP (with no antibody present, and confirmed by mutational analysis) or antibody mediated disease (with proven Anti-ADAMTS 13 antibodies)
IUGR, IUFD, PET, renal impairment
PEX required for acute presentation. Subsequent pregnancies require therapy throughout. Acquired TTP requires associated immunosuppressive therapy
HUS
Renal impairment or failure
Complement dysfunction
Dialysis, ESRF, hypertension
PEX, eculizumab
SLE
Thrombocytopenia, hemolytic anemia, pancytopenia, nephritis
ANA, dsDNA
Hypertension, preterm delivery, IUGR
Immunosuppression, symptomatic
APLS
Recurrent miscarriages or IUFD
LA, ACLA result in abnormal placentation
IUGR, IUFD, preterm delivery
LDA, LMWH, PEX
AFLP
Non-specific features, RUQ pain, GI hemorrhage, coagulation abnormalities/DIC, acute renal failure, pancreatitis, hypoglycemia, liver failure, encephalopathy
Fetal deficiency of LCHAD, a mitochondrial enzyme
Within hours of birth, the fetus presents with non-ketotic hypoglycemia, which can quickly progress to coma and death
Treat hypoglycemia. Genetic screening
17.2 Moderate to Severe Thrombocytopenia Presenting During Pregnancy
Thrombocytopenia is defined as a platelet count <150 × 109/L and results from increased destruction/consumption or decreased production. Thrombocytopenia can affect 10 % of pregnancies. However, a platelet count of under 100 × 109/L, as defined by an international working group [1], is encountered in 1 % of pregnant women. The challenge is to determine the underlying pathophysiology. The most common is gestational thrombocytopenia, occurring in 75 % of all cases, in which the platelet count is rarely below 70 × 109/L. It is characterised by a normal platelet count at the antenatal booking visit, is most apparent in the third trimester and returns to normal within 12 weeks postpartum. Gestational thrombocytopenia is thought to result from the hemodilutional effect of pregnancy and placental platelet destruction [2].
Idiopathic thrombocytopenic purpura (ITP) occurs in 5 % of pregnancies and is a result of peripheral platelet destruction [3]. Occasionally maternal treatment (oral steroids or IV immunoglobulin) and precautions during delivery (avoidance of fetal scalp blood sampling, fetal scalp electrode, ventouse delivery or difficult forceps delivery) may be required, but rarely does it have an affect on the fetus prior to labor and delivery. PET and HELLP account for 21 % of all cases of thrombocytopenia and the platelet count usually returns to normal within 3–5 days after delivery.
17.3 Acute Renal Injury in Pregnancy
Serum creatinine normally falls as pregnancy proceeds because of hemodilution, hyperfiltration and reduced oncotic pressures. Seventy-five percent of cases of renal impairment in pregnancy occur in the third trimester and postpartum. The leading cause of acute kidney injury in pregnancy is the hypertensive disorders of pregnancy; other causes include sepsis, hemorrhage, intrauterine fetal death (IUFD), AFLP and TMAs. For most of these differential diagnoses, pregnancy plays a primary role. However, in the case of TMAs, pregnancy is the trigger for a condition primarily associated with an underlying genetic risk. Acute renal injury may range from increased creatinine levels to dialysis dependence, and can be associated with significant feto-maternal morbidity and mortality.
17.4 Pre-eclampsia
17.4.1 Clinical Features
The definition of PET specifies de novo hypertension (i.e. blood pressure >140/90 mmHg) and proteinuria (>0.3 g/24 h) occurring after 20 weeks of gestation [4]. This definition encompasses the triad of hypertension, proteinuria and edema, but this is essentially a multisystem disorder that affects 5–10 % of pregnancies. PET is the second most common cause of thrombocytopenia in the second half of pregnancy and accounts for approximately 20 % of all cases. Platelet counts <50 × 109/L occur in less than 5 % of women with PET and, in the presence of intravascular hemolysis, other TMAs such as TTP must be excluded. Indeed, consideration should be given to checking ADAMTS 13 activity and giving treatment for TTP, if appropriate. Liver involvement in PET is the most common cause of hepatic tenderness and liver dysfunction in pregnancy. It is one of the indicators that should prompt consideration of delivery because of the high perinatal morbidity and mortality and the increased risk of eclampsia (seizures), hepatic rupture and necrosis. In 2–12 % of cases of eclampsia, the condition is complicated by HELLP syndrome. Other potential complications include renal impairment, stroke, placental abruption and abnormalities of the coagulation system. As with all the TMA’s, adverse affects on the placenta can result in fetal growth restriction and premature birth (usually as a result of medical intervention).
17.4.2 Serum Markers for Predicting Pre-eclampsia
The placenta is central to the pathogenesis of PET, and the only curative treatment is delivery and removal of the placenta, with symptoms usually improving within days. Failure of trophoblastic invasion of the maternal spiral arteries results in inadequate vascular remodelling. This in turn results in placental ischemia and infarction, which can be confirmed histologically. Uteroplacental ischemia is associated with the release of soluble factors that lead to widespread maternal endothelial dysfunction that manifest as the clinical features of PET. It is the disturbed balance between placenta derived anti-angiogenic factors and proangiogenic factors that are thought to contribute to the pathogenesis of PET [5, 6]. Placental ischemia can also cause IUGR, and PET and IUGR often present together. Therefore, early diagnosis and timely delivery are imperative for perinatal survival.
17.4.2.1 Placental Growth Factor (PIGF) and Soluble Fms-Like Tyrosine Kinase-1 (sFlt-1)
Fms-like tyrosine kinase-1 (Flt-1, soluble Flt (sFlt-1) and their ligands PIGF and vascular endothelial growth factor A (VEGF-A) are expressed by trophoblasts and their expression is altered in PET compared to normotensive controls. In PET, PIGF levels are reduced; serum concentrations of sFlt-1 and soluble endoglin (sEng) are increased, and the increase appears to correlate with disease severity. In a rodent model, overexpressing sFlt can trigger PET [7]. Furthermore, in other disease states, such as cancer, anti-VEGF therapy was associated with the development of hypertension and proteinuria [8].
In normal pregnancy, serum PIGF levels decrease and sFlt-1 levels increase in the third trimester. In PET, these changes are seen earlier in pregnancy and can be used to predict the disease 5 weeks before the onset of clinical symptoms. PIGF levels were lower before 12 weeks’ gestation in women who subsequently developed PET later in pregnancy [9, 10]. It has also been shown that women who have had a pregnancy affected by PET are at increased risk of cardiovascular disease (stroke, ischemic heart disease) later in life (see Sect. 17.4.7).
17.4.2.2 Placental Protein 13
The levels of placental protein 13 (PP13) are decreased in women who develop pre-eclampsia. The utility of a combination of uterine artery Doppler pulsatility index (PI) and maternal serum PP-13 measured in the first trimester (between 11 and 13 weeks) for the prediction of PET has been investigated [11]. PP-13 levels were lower and pulsatility indices higher in women that subsequently developed PET. This study had a high detection rate (90 %) for a false positive rate of 6 %.
17.4.2.3 Soluble Endoglin (sEng)
Endoglin is a co-receptor for transforming growth factors β1 and β3, expressed on trophoblasts. Its levels are increased in PET [12] and in pregnant rats this has been associated with increased vascular permeability and hypertension. When both sFlt-1 and sEng levels were elevated, symptoms were markedly worse, and associated with the development of HELLP syndrome. In a longitudinal analysis, the rise in sEng concentrations occurred earlier and was more marked in pregnancies in women that subsequently developed PET [13].
17.4.3 Management of Pre-eclampsia
The three main aspects of therapy are the management of hypertension (both systolic and diastolic), prevention and treatment of eclampsia, and careful fluid balance management. Delivery is the ultimate cure and particularly in women near term delivery may be appropriate, especially if there is associated HELLP syndrome. However, if anti-hypertensive treatment is required, one option is nifedipine, which is associated with reduced maternal hypotension. However, it should be avoided in women with known coronary heart disease or diabetes (for more than 15 years) or who are older than 45 years because of the risk of sudden cardiac death. Labetalol is less effective, but has a preferred side-effect profile compared to hydralazine. In severe PET, magnesium sulphate (MgSO4) should be used to reduce the risk of seizures. In general, timing of delivery depends on the maternal condition, fetal wellbeing (which can be assessed by umbilical artery Doppler), the gestational age and the neonatal care facilities available. Steroids for fetal lung maturation should be considered for pregnancies less than 36 weeks’ gestation.
Blood component support may be required, particularly in women with a coagulopathy. Platelets are not usually administered, but may be indicated if platelet counts are <50 × 109/L and particularly if the platelet count is dropping precipitously [14].
17.4.4 Antiplatelet Agents for the Prevention of Pre-eclampsia
Placental ischemia and infarction in PET is thought to be a result of platelet aggregation and activation of the coagulation system and platelets. Initial trials suggested that antiplatelet therapy might prevent PET [4, 15, 16]. The PARIS (perinatal antiplatelet review of International studies) collaboration undertook a systematic review and meta-analysis to assess the use of antiplatelet agents for the primary prevention of PET and to explore women most likely to benefit from such treatment [17]. There was a 10 % reduction in the relative risk of both PET (p = 0.004) and preterm delivery before 34 weeks (p = 0.011). However, there was no difference in antepartum haemorrhage (APH), postpartum haemorrhage (PPH) or placental abruption compared to controls. There was a 7 % reduction in the RR of preterm birth before 37 weeks (p = 0.003). The conclusion was that antiplatelet agents are associated with a reduction in the relative risk of PET, preterm birth before 34 weeks and serious adverse pregnancy outcomes. The PREDO (Prediction and Prevention of Pre-eclampsia) group which included 152 women with risk factors for PET and abnormal uterine artery Dopplers, showed that low dose aspirin (LDA) did not prevent PET unless started before 16 weeks’ gestation [18].
17.4.5 Future Therapeutic Targets
Over 50 proteins synthesised by the placenta have been investigated, and an understanding of their identities and role is being elucidated by genetic techniques including mRNA expression [19]. sFlt-1 ligands such as VEGF or PIGF have been shown in animal studies to improve PET [20]. More recently, it has been shown that low molecular weight heparin (LMWH) increases levels of both circulating and urinary sFlt-1; and that heparin bound sFlt-1 has decreased affinity to negatively charged surfaces when compared to sFlt-1 alone. It is speculated that upon heparin treatment, sFlt-1 bound to heparan sulfate proteoglycans of the extracellular matrix are mobilized into the circulation [21]. Furthermore, in a meta-analysis of randomized controlled trials comparing prophylactic LMWH with no therapy, there was a significant relative risk reduction in adverse pregnancy outcomes (18.7 % vs 42.9 % in treated and non-treatment groups, respectively). These adverse outcomes include PET, severe PET, small for gestational age (<10 % percentile), preterm delivery <37 weeks and preterm delivery <34 weeks. Therefore, LMWH may be a useful therapy for placenta mediated pregnancy complications but further trials are required [22]. Statins can upregulate pro-angiogenic factors and also improve PET in animal models [23].
17.4.6 Outcome of Subsequent Pregnancy After First Pregnancy with Early Onset PET
Presentation with PET is generally near term, and much more common in nulliparous women. However, 10 % of women who develop PET will present before 34 weeks. Earlier presentations are associated with worse outcomes for both mother and fetus. Recurrence rates in subsequent pregnancies are variably quoted between 15 and 65 % [24]. Treatment in subsequent pregnancies with LMWH and LDA has been reported to improve outcomes [25]; LMWH is thought to have a dual role as an anti-inflammatory and anti-thrombotic agent. Furthermore, women with a history of chronic hypertension also had an improved outcome with this therapy. More recently, using LDA alone before 16 weeks’ gestation reduced the risk of PET and severe PET in high-risk women [17, 18].
17.4.7 Long-Term Effects of Pre-eclampsia
Later in life, women with a history of PET have an increased risk of cerebrovascular complications such as hypertension, ischemic heart disease and stroke, that is thought to stem from endothelial dysfunction [26, 27]. This risk appears to be greater in women who have preterm PET or PET complicated by IUGR [28]. There is an increased risk of peripartum cardiomyopathy and it has been suggested that the babies are at increased long-term risk of pulmonary hypertension. There is also a suggestion of an increased relative risk of end stage renal failure, although the absolute risk remains low [26]. What remains unclear is whether the long-term cardiovascular risks associated with PET are due to damage caused by the pre-eclamptic process, or whether women who develop PET have a pre-existing cardiovascular phenotype that predispose them to both PET and later cardiovascular disease.
17.5 Thrombotic Thrombocytopenic Purpura
17.5.1 Clinical and Laboratory Features
TTP is an acute life-threatening disorder associated with thrombocytopenia, microangiopathic hemolytic anemia and symptoms related to microvascular thrombosis. Clinically, in addition to a low platelet count (<150 × 109/L, but typically <50 × 109/L), patients are anemic secondary to hemolysis, with acute consumption of folate. Corresponding blood film changes include polychromasia, anemia, reduced platelets and fragmented red blood cells. Bilirubin is often raised but the direct antiglobulin test is negative and the clotting screen is normal. Lactate dehydrogenase (LDH) is increased, often to a greater extent than the degree of hemolysis, due to associated tissue ischemia [29].
17.5.2 Pathophysiology
von Willebrand factor (vWF), a plasma glycoprotein synthesized by megakaryocytes and endothelial cells, normally circulates as multimers of 500–20,000 kDa. Ultra-large vWF multimers (ULvWFM), >20,000 kDa, not normally detected in plasma, have been detected in patients with chronic relapsing TTP [30]. The deficiency of vWF-cleaving protease in patients with TTP has been identified as a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13’ or ADAMTS 13 [31, 32]. This enzyme is required to break down ULVWF multimers, and failure to do so, due to an inherited deficiency of ADAMTS 13 (in congenital TTP) or an acquired reduction (due to antibodies to ADAMTS 13), leads to platelet adhesion and aggregation on ULvWF multimers, which results in microvascular thrombosis. Hence, platelet transfusions are contraindicated in TTP, as infusions potentiate the effects of platelet aggregation on ULvWF multimers.
As a precipitating cause of acute TTP, pregnancy accounts for only a small proportion of all cases in women [33, 34]. TTP is more common in women than in men (3:2) and 45 % of all cases occur in women of childbearing age. There is also the risk of relapse of TTP during subsequent pregnancies once women have been diagnosed. Other pregnancy related thrombotic microangiopathies, such as PET/HELLP (hemolysis, elevated liver enzymes, low platelets) and hemolytic-uremic syndrome (HUS) may further complicate the diagnosis of TTP but may also be part of the TTP syndrome.
17.5.3 Hemostatic Changes of Normal Pregnancy: Factor VIII, von Willebrand Factor (vWF) and ADAMTS 13
Normal pregnancy is associated with marked prothrombotic changes in hemostasis that are hormonally mediated and protect against severe hemorrhage at the time of birth, but result in a hypercoagulable state. Factor VIII and vWF increase in parallel in the first half of pregnancy; thereafter, the increase in vWF is greater throughout the remainder of pregnancy, returning to normal levels over the 6 postpartum weeks. Reciprocal changes of vWF and ADAMTS 13 have been documented. Therefore, with the increased vWF in pregnancy, ADAMTS 13 would be expected to decrease.
A review of ADAMTS 13 in normal women with no history of TTP documented a reduction in ADAMTS 13 activity in the 2nd and 3rd trimesters of pregnancy [35]. Another study in healthy women confirmed a reduction in ADAMTS 13 activity after the first trimester (weeks 12–16) up until the end of the postnatal period, when levels normalised to pre-pregnancy levels. Interestingly, this group found that ADAMTS 13 activity was lower in non-pregnant nulliparous women (mean 65 %) compared to parous women (mean 83 %). In pregnancy and postpartum, mean ADAMTS 13 activity was slightly, but non-significantly, lower in primigravidae than in multigravidae (68 % vs 74 %). ADAMTS 13 was unaffected by platelet count, but was significantly higher in smokers than in non-smokers during pregnancy (mean 79 % vs 70 %, respectively). There was a significant inverse correlation between vWF: Ag levels and ADAMTS 13 activity [36]. The cause of the decrease in ADAMTS 13 during pregnancy may be twofold. Firstly, enzyme levels decrease with excess substrate, namely vWF. Secondly, a hormonal influence, possibly estrogen, may reduce ADAMTS13 levels.
17.5.4 Women Presenting with Acute TTP During Pregnancy
Women presenting with TTP during pregnancy appear to fall into two groups; those with congenital TTP and those with acquired, antibody mediated TTP. Congenital TTP may first present during pregnancy; indeed, it is more likely that presentation in pregnancy will be congenital TTP [37, 38] and these women will relapse in subsequent pregnancies. Diagnosis of congenital TTP is confirmed in patients with ADAMTS 13 activity <5 %, no evidence of an inhibitor and confirmation by mutational analysis of the ADAMTS 13 gene, revealing a homozygous or compound heterozygous abnormality.
17.5.5 Risks in Pregnancy in Women with Previous Acquired Idiopathic (Non-pregnancy Associated) TTP
A particular concern in women who have had acute TTP unrelated to pregnancy is the risk of relapse during pregnancy. This occurs in 30–50 % of cases and the risk of fetal loss is significant. In those patients in whom ADAMTS 13 was tested, normal levels pre-pregnancy or at the onset of pregnancy were associated with a lower likelihood of relapse. Another important feature is the associated complications in TTP, such as pre-eclampsia and HELLP. Thrombotic microangiopathies during pregnancy may be clinically indistinguishable from one another and very difficult to treat. Because the reduction in ADAMTS 13 levels occurs from the onset of the second trimester, it had originally been proposed that this was the time of increased presentation of acute TTP. However, it now appears that the time of greatest risk is in the 3rd trimester or postpartum.
17.5.6 Management of TTP in Pregnancy
The primary decision is whether delivery will be associated with remission of the TMA (as it is in PET and HELLP) or whether plasma exchange (PEX) should be instigated, if recovery following delivery is unlikely and there is a risk of multi-organ dysfunction or death. A further complicating issue is the possible development of HELLP/PET following delivery, which may occur in 20–30 % of cases. If TTP develops in the first trimester, PEX may allow continuation of pregnancy with delivery of a live infant. However, in contrast to PET and HELLP, delivery does not necessarily induce remission of TTP [39–41]. Later in pregnancy, differentiation from other pregnancy associated TMAs can be very difficult, but is important. Delivery is the definitive treatment of choice for pregnancy associated TMAs and, with recovery after delivery, TTP can be excluded. If there is progression of symptoms despite delivery, PEX is the most appropriate option.
A prospective study of TTP cases from the United Kingdom TTP Registry with clinical and laboratory data from the largest cohort of pregnancy-associated TTP [42] describes management through pregnancy, averting fetal loss and maternal complications. The study included 35 women who presented with a first TTP episode during pregnancy: 23/47 pregnancies with their first congenital TTP (cTTP) episode and 12/47 with acute acquired TTP in pregnancy. The conclusions from this study are that careful diagnosis, monitoring, and treatment in congenital and acquired TTP have assisted in excellent pregnancy outcomes [42].
With the availability of ADAMTS 13 activity measurement and detection of inhibitors to ADAMTS 13 or more specifically IgG antibodies, these markers may be helpful to distinguish TTP from other pregnancy associated TMAs. Specifically, if ADAMTS 13 activity is under 5 % and/or there are anti-ADAMTS 13 IgG autoantibodies, it is diagnostic of TTP. In HELLP, ADAMTS 13 activity is reduced (median 31 %, range 12–43 %) but with no inhibitor/antibodies to ADAMTS 13 and higher VWF levels [43]. Women presenting with thrombocytopenia, microangiopathic haemolytic anaemia (MAHA), neurological features (such as stroke/TIA, seizures, encephalopathy) or renal impairment, should be treated with PEX until the diagnosis of TTP is excluded.
Steroids may be useful in TTP and HELLP, but for differing reasons. They have been empirically used in TTP because of the underlying autoimmune basis of the disorder. In HELLP, steroids may accelerate platelet recovery and pre-delivery, dexa-/betametasone will aid fetal lung maturity.
In women with congenital TTP, the risk of relapse in subsequent pregnancy is such that elective plasma therapy, to provide ADAMTS 13 supplementation, throughout pregnancy and the post-partum period, is warranted. Plasma infusions may be satisfactory; however, in order to deliver sufficient volumes, PEX may be required. The precise optimal frequency of plasma replacement is unknown; the half-life of ADAMTS 13 is 2–3 days [44] and plasma therapy every 2 weeks appears satisfactory. However, this often needs to be increased to weekly from the 2nd trimester, and the appropriate frequency can be determined by monitoring platelet counts and LDH.
In women with acquired TTP, it is not as easy to predict which patients are likely to relapse and the literature is sparse in this area. A previous history of TTP and ADAMTS 13 activity at the onset of pregnancy may be helpful in differentiating patients most likely to relapse in subsequent pregnancies. A normal ADAMTS 13 at the onset of pregnancy appears to predict women at reduced risk of subsequent relapse [45]. However, low ADAMTS 13 activity (<5 %) prior to pregnancy should prompt consideration of elective therapy to prevent relapse. In contrast, women with normal ADAMTS 13 activity at the onset of pregnancy who maintain normal routine laboratory parameters, ADAMTS 13 activity and antibody/inhibitor levels throughout pregnancy, do not usually require intervention for TTP. A reduction in ADAMTS 13 activity (<10 %) may be the catalyst to instigate elective therapy to prevent microvascular thrombosis during pregnancy.
Adjunctive therapy during pregnancy has not been addressed in the literature; specifically, LDA and/or prophylactic LMWH administration. LDA throughout pregnancy should be considered, and women with a documented thrombophilia or a past history of venous thromboembolism associated with TTP, may require prophylactic LMWH. The aim is to optimize implantation and preserve placental function, as abnormalities of the uteroplacental circulation resulting in placental insufficiency are established in the first trimester [46]. LDA/LMWH may be beneficial in other thrombophilia disorders during pregnancy due to the effects of placental abnormalities secondary to infarction. However, this therapy has not been formally evaluated in pregnancy associated TTP. There are no data on the microvascular effects of ‘subacute’ TTP prior to presentation with thrombocytopenia. Therefore, women with a previous pregnancy loss due to TTP or low ADAMTS 13 activity at the onset of pregnancy can be assumed to be at increased risk of further episodes in subsequent pregnancies. Women who have a past history of TTP may have an increased risk of complications, such as pre-eclampsia, which could in part be attributable to placental infarction due to thrombotic occlusion of the decidual arterioles. Supportive measures may help to reduce such complications in those at risk. Red cell transfusion should be administered according to clinical need especially if there is cardiac involvement. Due to the risk of precipitating further thrombotic events, platelet transfusions are contraindicated unless there is life- threatening haemorrhage [29].
In summary, women with congenital TTP require therapy with plasma, either as infusions or PEX to provide ADAMTS 13 supplementation, throughout pregnancy and the post-partum period. In women with acquired TTP and previous acute TTP, the baseline ADAMTS 13 activity and inhibitor/antibody status may be useful in the identification of those most likely to relapse. Monitoring of enzyme activity in those with normal pregnancy levels may be useful, but in women with low (<5 %) ADAMTS 13 activity and/or raised IgG antibody levels, who appear to be at increased risk of relapse during pregnancy, elective PEX may be useful. Adjunctive therapy with LDA in all women +/− prophylactic LMWH should be added to help prevent complications related to placental thrombosis. Close liaison with an obstetrician with a special interest in feto-maternal medicine is required in mothers with TTP. Pre-conceptual counselling is advised for subsequent pregnancies and women of child bearing age should be counselled about potential risks of pregnancy and COCP.
17.6 Liver Disease in Pregnancy
Clinically relevant changes in liver function are seen in only 3–5 % of all pregnancies and in the majority pregnancy is the primary precipitant. In the first trimester, hyperemesis gravidarum is the main cause; in the 2nd and 3rd trimesters, the primary culprit is intrahepatic cholestasis of pregnancy.
17.6.1 Intrahepatic Cholestasis of Pregnancy
17.6.1.1 Clinical and Laboratory Features
The first feature of intrahepatic cholestasis of pregnancy (ICP) is usually pruritus (10–25 % of patients), followed by jaundice and a 10–20-fold increase in aminotransferases (bilirubin is not usually raised to the same extent). The diagnosis is confirmed by measuring bile acid levels. The ultimate treatment is delivery, but management in the interim (in order to gain fetal maturity) is with ursodeoxycholic acid (UDCA), which can both improve symptoms of itching and reduce levels of bile acids and aminotransferases. Steroids have not been shown to be useful in managing ICP (although may be used to improve fetal maturity when preterm delivery because of the ICP is anticipated. Raised bile acid levels may be associated with placental insufficiency. The condition resolves with delivery, but recurrence is seen in approximately 50 % of cases in future pregnancies or with the use of the combined oral contraceptive pill. Underlying abnormalities of progesterone are thought to be the pathological defect [47].
17.6.2 Acute Fatty Liver of Pregnancy
17.6.2.1 Clinical Features
AFLP is a rare disorder the incidence of which is estimated at 1 in 13,000 births, but it is an acute life-threatening illness associated with significant maternal and perinatal mortality [48]. It presents primarily in the third trimester, usually affecting women in their first pregnancy, but recurrence in subsequent pregnancies has been reported.
Clinically, the presentation is non specific, with headache, fatigue, nausea, vomiting (70 %) and right upper quadrant or epigastric pain (50 %). There may be gastrointestinal hemorrhage, coagulation abnormalities, acute renal failure, infection, pancreatitis and hypoglycemia early in the presentation. Usually there is progression to liver failure and hepatic encephalopathy. Improvement occurs 1–4 weeks post-partum.
17.6.2.2 Diagnosis
The diagnosis is usually clinical but, if there is uncertainty, may be confirmed by liver biopsy. Histologically, in the liver there is characteristic microvesicular steatosis and, with Oil Red O staining, cytoplasmic vesiculation as a result of microvesicular fat. However, it is often not possible to undertake a liver biopsy if coagulation is affected because of the increased risk of hemorrhage.
Laboratory testing reveals a raised white cell count and thrombocytopenia, with evidence of DIC (with prolonged prothrombin time (PT), activated partial thromboplastin time (APPT) and reduced fibrinogen). Urea, creatinine and uric acid levels are raised, as are ammonia levels, and there is hypoglycemia. Serum aminotransferases are raised and alkaline phosphatase is three to four times higher than normal. The primary differential diagnoses are acute fulminant hepatitis and severe HELLP syndrome, although these conditions are less likely to feature hypoglycemia or a prolonged PT.
17.6.2.3 Pathophysiology
The pathophysiology of AFLP is interesting. This condition results from mitochondrial dysfunction, associated with a deficiency of the enzyme long chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) in the fetus. This enzyme is important in mitochondrial fatty acid β-oxidation [49]. β-oxidation of fatty acids is a major source of energy for skeletal muscle and the heart. The liver oxidizes fatty acids under conditions of prolonged fasting, during illness and at periods of increased muscular activity. Mitochondrial β-oxidation of fatty acids is a complex process. LCHAD is part of an enzyme complex, the mitochondrial trifunctional protein (MTP), which is associated with the inner mitochondrial membrane [49, 50]. Defects in the MTP complex are recessively inherited and are due to an isolated LCHAD deficiency, specifically associated with a G1548C mutation. A few hours after birth, the newborn presents with non-ketotic hypoglycemia and hepatic encephalopathy, progressing to coma or death, if untreated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree