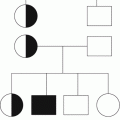
Variable
GT
ITP
Gestational age at diagnosis (weeks)
32.8 ± 7.6
20.1 ± 11.1
Gestational age when platelet count counts at nadir (weeks)
35.7 ± 5.5
27.0 ± 11.2
Platelet count at diagnosis (×109/L)
71.3 ± 19.2
40.0 ± 25.5
Platelet count at nadir (×109/L)
58.6 ± 24.1
24.3 ± 15.5
Platelet count on day of delivery (×109/L)
70.1 ± 28.2
37.3 ± 24.0
Platelet count on day 3 postpartum day 3 (×109/L)
100.9 ± 1.6
81.9 ± 26.3
The incidence of GT has been investigated by several authors. In two population studies, one including almost 7,000 and the other over 4,000 women, the rate of GT was 10.9 and 5.9 %, respectively, while in another study it was estimated at 5.4 % [2–6, 8]. Although the pathogenesis of this phenomenon is unknown, it has been proposed that thrombocytopenia results from the relative hemodilution that occurs in pregnancy in combination with the capture and destruction of platelets in the placental bed [11]. Women with GT are usually asymptomatic and do not have a history of previous thrombocytopenia, although if they have previously been pregnant, are likely also to have had GT in the previous pregnancy. The diagnosis is usually made when a low platelet count is discovered incidentally on routine laboratory testing during the second or third trimester, or when delivery is imminent [2–6]. Other hematological and biochemical parameters are usually normal [2–6].
In the majority of women with GT, pregnancy and the peripartum period are uneventful, and there are no complications for either mother or baby related to the low platelet count [2–6, 9]. The risk of developing a low platelet count in neonates of mothers with GT has been determined from cord blood samples and was found to be 4 %, similar to that observed in infants born to women with a normal platelet count [2–6]. However, in a prospective study of cord blood platelet counts in women with thrombocytopenia, 7 of 33 (21 %) babies of mothers with GT had low platelet counts (none of the babies had sepsis). The neonates’ platelet counts ranged from 58 to 144 × 109/L, and none required treatment. The authors recommended that for every baby born to a mother with a pregnancy-associated thrombocytopenia, even in the case of confirmed GT, platelet count in cord blood should be checked [12]. Although GT is a benign condition, platelet counts should be monitored during pregnancy in order to detect any sudden decline with the potential for subsequent hemorrhagic manifestations [2–6].
15.3 Immune Thrombocytopenic Purpura (ITP): Definition
Immune thrombocytopenic purpura is an acquired disorder of platelet number that is characterized by immune-mediated platelet destruction [2–6, 13].
The disease is usually benign with the majority of patients being asymptomatic [3–6]. It is not infrequent in women of childbearing age, although the prevalence increases with age [14–16]. The main clinical feature of the disease is hemorrhagic manifestations ranging from mild cutaneous bleeding lesions such as petechiae and ecchymoses to, rarely, severe intracranial hemorrhage [2, 13, 17, 18]. Although in children the disease is usually self-limiting, in adults it usually runs a relapsing-remitting course [2, 17, 18].
15.4 ITP: Terminology and Classification
An international working group on ITP stipulated that its diagnosis requires a platelet count lower than 100 × 109/L [18] and that the thrombocytopenia should be isolated, with an otherwise normal full blood count, peripheral blood smear and biochemical indices [18]. Additionally, on clinical examination there should be no pathological features indicative of an underlying disease that could precipitate thrombocytopenia [18]. In borderline conditions, when the platelet count ranges between 100 and 150 × 109/L, patients have a low probability (6.9 %) of developing more profound thrombocytopenia and hemorrhagic complications [18]. These patients should be regularly monitored but they do not usually need treatment [18–20]. ITP is termed ‘persistent’ when it lasts between 3 and 12 months, and ‘chronic’ when low platelet counts are observed for more than 12 months [18]. It is very important to distinguish between primary and secondary ITP [18]. Secondary ITP has a different natural history and usually resolves when the underlying condition is treated [18].
The same definitions should be applied to pregnant women with ITP [21].
15.5 ITP: Epidemiology
The incidence of ITP is not well defined. In a study based on the UK General Practice Research Database, the incidence of ITP in the general population has been determined as 3.9 cases/100,000 person-years. The incidence of ITP in women ranges from 3.7 cases/100,000 person-years in those under 18 years to 3.8 between 18 and 64 years and 7.1 in women above 65 years [14–16].
During pregnancy, determination of the incidence of ITP is even more challenging because of the difficulty in distinguishing ITP from other causes of thrombocytopenia, including GT [2–6]. Some authors estimate the prevalence of ITP as approximately 1–5 cases/10,000 pregnancies, whereas the prevalence of GT in the same population is 100 times greater [2–6, 22–24]. Other investigators estimated that ITP occurs in 1/1,000–1/10,000 pregnant women [2–6, 14, 21–23]. In a Korean study, among 31,309 women who were reviewed, 25 (0.07 %) were diagnosed with ITP and 33 (0.1 %) were diagnosed with GT [10].
Similar results were obtained from a study that enrolled 62,441 pregnant women: The diagnosis of ITP was established in 55 women (0.08 %), 24 of whom had a previous history of ITP [25].
15.6 ITP: Pathophysiology
Although the underlying pathophysiological disorder in ITP remains unknown, several studies convincingly demonstrate an altered immune response, as reviewed by Cines and Mc Millan [26]. The major feature of ITP has been considered to be the production of anti-platelet antibodies (IgG or IgM) by activated B lymphocytes. The target of these antibodies is most frequently platelet membrane glycoproteins GPIIb/IIa and Ib/IX [26, 27], although other platelet membrane glycoprotein antibodies have also been described [26, 27]. Immune complexes containing platelets coated by antiplatelet antibodies are cleared by the Fcg receptors of the mononuclear cells in the reticuloendothelial system [28]. These cells are located primarily in the spleen but also in other organs such as the liver and bone marrow [28, 29]. Specific genetic polymorphisms of the Fcg receptors have been observed in patients with ITP, which may result in enhanced clearance of the antibody-coated platelet [27–31]. Although antiplatelet antibodies had been considered the hallmark of ITP, they have characteristically been difficult to measure and it is clear that T lymphocytes contribute to, if not initiate, ITP pathogenesis [26, 29, 32–34]. A number of studies have described polarization of T helper cells toward the Th1 immune response with an increase in the production of IL-2, IFN-g, and TNF-a [26, 29, 32–34]. This response activates cytotoxic, inflammatory and delayed hypersensitivity reactions [26, 29, 32–34]. In contrast, there is a reduction in Th2 response cells that produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 cytokines [26, 28, 29, 31–34]. Activated T cells not only increase the biogenesis of B cells producing antiplatelet antibodies but also show direct CD8-mediated cytotoxicity against platelets and megakaryocytes in some patients with ITP [32, 35]. More recently, regulatory T cells have been found to be decreased in both number and functional activity when compared to those in healthy individuals, indicating loss of immune tolerance [36, 37]. Ongoing interaction between B cells and T cells is also suggested by elevated CD40 and CD40L (CD154) on the surface of B and T cells. Increased levels of CD154 were also found on the platelet surface in patients with ITP, suggesting that in vivo activation of autoreactive B lymphocytes may be driven by platelets [38].
There are also signals that impaired thrombopoiesis contributes to the thrombocytopenia in ITP [21, 26, 29, 39]. Platelet survival studies have revealed a low or inappropriately normal (rather than increased as a response to thrombocytopenia) production of platelets in patients with ITP [21, 26, 29, 39, 40]. Electron microscope images and in vitro studies suggest increased apoptotic indices and poor differentiation, presumably driven by antiplatelet antibodies directed at the megakaryocytes [41]. In addition, it has been reported that, in bone marrow biopsy specimens from ITP patients, there is an increased proportion of megakaryocytes with activated caspase-3, reflecting a direct effect of B and T cells on megakaryocytes [42].
Moreover, thrombopoietin, the growth factor for megakaryocytes and platelets, is inappropriately low for the platelet count when compared to the thrombopoietin levels in patients with thrombocytopenia due to other causes such as aplastic anemia [21].
Although in the majority of patients with ITP the precipitating cause remains obscure, it has been observed that viral and bacterial infections may initiate the condition [26, 29, 43]. Viruses may cause secondary ITP, inducing loss of immune tolerance, a decrease in T helper cells, direct proliferation of B cells leading to increased antibody production, and macrophage activation which contributes to the disease severity [26, 29]. Bacterial infections, such as H. pylori infection, are also implicated in the pathogenesis of ITP, and eradication therapy can result in prolonged remissions [43]. In this situation ITP may be caused by molecular mimicry where, instead of additional production of antibodies against H. pylori, there is antibody production to self. Moreover, alterations in the cytokine milieu stimulate B cell activation and loss of immune tolerance [26, 29, 43].
Overall, it is clear that ITP is characterized by pathogenetic diversity. Several factors can provoke the initiation of the disease and contribute to thrombocytopenia. This pathogenetic diversity observed in ITP leads to similar variability in the clinical expression of the disease. The variation observed in the clinical picture is irrespective of the severity of thrombocytopenia and the presence of antiplatelet antibodies [17].
Better understanding of the pathogenesis of the disease will hopefully lead to treatments targeted at many of these pathophysiological alterations, and amelioration of the hemorrhagic tendency [29].
15.7 ITP: Clinical Features and Differential Diagnosis
As also occurs in the general population with ITP, pregnant women with ITP may present with hemorrhagic manifestations of variable severity, or thrombocytopenia might be an incidental finding on routine laboratory testing. There may be a preceding history of ITP with or without treatment [2–6, 13, 44].
Women with a history of previous ITP may present with an exacerbation during any trimester, or the disease may remain quiescent [3–6]. As in the non-pregnant population, the diagnosis of ITP is one of exclusion [17, 22, 45]. In accordance with the International Consensus Report guidance on the diagnosis and treatment of ITP, it is important that other causes of maternal thrombocytopenia during pregnancy should be excluded [13, 17]. In Table 15.2, the causes of thrombocytopenia associated only with pregnancy are shown. Thrombocytopenic conditions not necessarily associated with pregnancy are listed in Table 15.3 and laboratory investigations that should be performed in pregnant women with suspected ITP for the exclusion of underlying diseases are shown in Table 15.4. Serum antiplatelet antibodies cannot clearly establish the diagnosis of ITP because they lack specificity. During pregnancy, they are equally uninformative and they do not predict the risk of maternal or fetal hemorrhage [17, 44]. In a study including 6,770 pregnant women, 566 of whom had thrombocytopenia, anti-platelet antibodies detected using the monoclonal antibody-specific immobilization of platelet antigens assay (MAIPA), were identified in 6.7 % of thrombocytopenic women; this was no different to the incidence of detection of antibodies in non-thrombocytopenic women [46]. Other investigators found that antiplatelet antibodies were evident at a similar rate of 20.8 % of pregnant patients with ITP and 16.7 % of those with GT [10]. Similarly inconclusive results have been reported in other studies [8, 25]. In this context, the International Consensus Report does not recommend serum antiplatelet antibody testing as is not considered to be a valuable method to establish the diagnosis of ITP in pregnancy [13, 17]. Similarly, bone marrow examination is not necessary for the confirmation of ITP in pregnancy, unless there are clinical or laboratory indications of another underlying hematological disease [13, 17]. In patients who fail to respond to conventional therapeutic strategies, or when splenectomy is planned for curative purposes, bone marrow aspiration and biopsy should be considered [13, 17].
Table 15.2
Causes of thrombocytopenia exclusively associated with pregnancy
Gestational thrombocytopenia |
Pre-eclampsia |
HELLP syndrome |
Obstetric hemorrhage |
Acute fatty liver of pregnancy |
Table 15.3
Causes of thrombocytopenia not necessarily associated with pregnancy
Spurious – EDTA-dependent platelet agglutination |
Drug-related thrombocytopenia (e.g., unfractionated heparin) |
Thrombotic microangiopathies (HUS, TTP, DIC) |
SLE and antiphospholipid syndrome |
Autoimmune diseases (autoimmune hepatitis, Crohn’s disease, thyroiditis) |
Nutritional deficiencies (folate and B12 deficiency) |
Lymphoproliferative diseases (CLL and lymphomas) |
Infections (HIV, HCV, H. Pylori) |
Immunodeficiencies (common variable immunodeficiency, IgA deficiency) |
Nutritional deficiencies (folate and B12 deficiency) |
Concurrent bone marrow disorders |
Congenital platelet disorders |
Type II von Willebrand Disease (IIB vWD) |
Hypersplenism |
Table 15.4
Investigation of suspected immune thrombocytopenic purpura (ITP)
Full blood count and peripheral blood film |
Coagulation screen (PT, APTT, fibrinogen) |
Liver function tests |
Antinuclear antibodies (ANA), anti-DNA and ENA |
Blood RhD group (if anti-D contemplated) |
HIV and HCV |
Antiphospholipid antibodies (lupus anticoagulant, anticardiolipin and anti-beta 2 glycoprotein 1 antibodies) |
15.8 ITP: Risks of ITP During Pregnancy
Although sometimes difficult to achieve, it is critical to determine the cause of thrombocytopenia, because women with GT usually have an uneventful pregnancy and peripartum period. In contrast, women with ITP sometimes develop severe thrombocytopenia and experience hemorrhagic complications during delivery and postpartum, and require more careful monitoring [2, 13, 17]. The platelet count is an important predictor of hemorrhage in these patients [2, 13, 17].
The risk of significant hemorrhage is related to the severity of the thrombocytopenia and the gestational age at the time of diagnosis of ITP [2, 13, 17]. Women with thrombocytopenia also have a higher rate of non-hemorrhagic maternal and fetal complications. In one study which compared 199 pregnant women with thrombocytopenia due to a variety of causes (mainly GT and to a lesser extent ITP, pre-eclampsia, DIC, HELLP syndrome, and antiphospholipid syndrome APS), to 201 women with normal platelet counts, the following complications were significantly more common (p < 0.001) in the thrombocytopenia group: preterm delivery (<37 weeks), placental abruption, intrauterine growth restriction (p < 0.003), stillbirth, need for induction of labor, low neonatal Apgar scores (<7) at 1 and 5 min, and need for blood or blood component transfusion in the mother [11].
15.9 ITP: Management During Pregnancy
The management of ITP in pregnancy should be undertaken within a multidisciplinary setting with close liaison between obstetricians, haematologists, anesthetists and neonatologists. Management is based on the estimated risk of significant maternal hemorrhage [2, 13, 17, 47]. As the platelet count usually declines during the third trimester, careful monitoring is required to ensure that it is adequate around the time of delivery [2, 13, 17]. The frequency of platelet count determination is based on the absolute number of platelets and the gestation [13, 17].
In patients with secondary ITP because of hepatitis C (HCV) infection, antiviral therapy should be considered. However, the platelet count should be closely monitored because of the risk of worsening thrombocytopenia attributable to interferon. For patients with HIV-associated ITP, treatment of the HIV infection with antiviral therapy should be considered before other treatment options unless the patient has clinically significant bleeding complications. H pylori eradication therapy should be administered to patients found to have this infection [11].
Women with ITP should receive preconception counseling. Counseling of ITP patients considering pregnancy should address safety of mother and fetus, outcomes of worsening maternal disease, and risks of pregnancy itself [17].
15.10 ITP: Indications for Treatment
Throughout the first and the second trimesters, treatment for ITP is not indicated unless:
Hemorrhagic manifestations are evident
The platelet count is lower than 20–30 × 109/L
There is a need to increase the platelet count to a level safe for an invasive procedure such as amniocentesis
During the second and the third trimesters or when delivery is imminent, the platelet count should exceed 20 × 109/L [2, 17, 48, 49]. There are some variations in guidance on platelet count thresholds around delivery, summarized here. The 2010 International Consensus Report states that hematologists believe that the platelet count considered safe for Cesarean section (CS) is at least 50 × 109/L (which is therefore also required for vaginal delivery as emergency CS may be required); and that obstetric anesthetists generally recommend a platelet count of at least 75 × 109/L for spinal or epidural anesthesia. The authors of the 2011 American Society of Hematology (ASH) guidelines state that they found no evidence has been found to support specific platelet count thresholds that are ‘safe’ in the antenatal or peripartum periods [1, 17, 48, 49].
15.11 ITP: Recommended Medical Treatment
Therapeutic options used for the treatment of ITP in pregnancy are similar to those used outside of pregnancy [2, 13, 17]. Drugs of choice for first-line treatment are corticosteroids and intravenous immunoglobulin (IVIg). There is also limited evidence supporting the use of intravenous (IV) anti-RhD immunoglobulin (anti-D) [2, 13, 50]. Splenectomy, azathioprine, or combinations of the first-line treatment options mentioned above can be used in unresponsive or relapsing patients [2, 13, 17].
Other therapeutic modalities such as rituximab, vinca alkaloids, danazol, recombinant thrombopoietin receptor agonists, and immunosuppressive drugs should generally be avoided during pregnancy because of lack of evidence of benefit and the risks of possible harmful effects on the fetus [17].
15.11.1 Corticosteroids
Prednisolone is the most commonly used therapy in both pregnant and non-pregnant patients [2, 13, 17]. Although the standard therapy for ITP is oral administration of prednisolone in a dose of 0.5–1 mg/kg/day [2, 13, 17], during pregnancy a lower dose, such as 10–20 mg a day, is frequently used [17]. Prednisolone is extensively metabolized in the placenta with only 10 % reaching the fetus, so is considered safe for both mother and fetus [4, 13, 17].
Concerns about the use of steroids include hypertension, hyperglycemia, osteoporosis, excessive weight gain and psychosis in the mother. If higher doses are used for initial therapy, the dose should be carefully tapered to the minimum required for maintaining a safe count for delivery [2, 22, 51].
Corticosteroid administration has been described in an observational study including 110 women, 37 of whom required therapy [24]. Prednisone alone was administered in eight patients, and in seven the drug was administered in combination with IVIg [24]. Treatment was effective in increasing the platelet count, but the increase was transient. No severe side-effects were observed [24]. In another study in 284 pregnant women, 94 required therapy. Eighty-five patients received corticosteroids in various dose regimens [52]. Women treated with doses exceeding 15 mg/day delivered infants with abnormal body weight, either small or large for dates (p = 0.017) [52]. Although corticosteroids are considered safe therapy for the fetus, a slightly increased incidence of fetal death and congenital abnormalities (p = 0.043) was observed in one group of patients treated with larger doses (exceeding 15 mg/day) compared with the non-treated patients [52]. It seems sensible, therefore, that the lowest dose of steroids required to maintain a “safe” platelet count should be used [24, 44, 52].
Platelet counts should be carefully monitored during pregnancy and after delivery, particularly during the phase of dose tapering, to avoid a rapid fall in levels [17].
15.11.2 Intravenous Immunoglobulin (IVIg)
When corticosteroid therapy is ineffective, or has unacceptable side effects, or high dose and prolonged duration of therapy is required to achieve an adequate platelet count, IVIg should be considered as an alternative [50, 53, 54]. As second-line treatment after steroid failure, IVIg should be administered when the platelet count falls below 10 × 109/L. IVIg should be administered, alone or in combination with corticosteroid, whenever a rapid rise in platelet count is needed, such as when the platelet count is 10–30 × 109/L and there is active bleeding, or in asymptomatic women with a platelet count 10–30 × 109/L when delivery is imminent [17, 22, 48].
There are no studies comparing the safety and efficacy of prednisolone and IVIg [53, 54]. However, data from observational studies on the administration of IVIg during pregnancy show that response rates are similar to those in the non-pregnant population, with an excellent safety profile for both mother and fetus [50, 53]. Although the response rate in pregnancy exceeds that with corticosteroids, with an increase in the platelet count of up to 80 %, the effect is usually transient [53].
The mode of activity of IVIg in ITP is complicated and relates to increased expression of the inhibitory receptor FcRIIb, possibly just from the activity of a small fraction of the IVIg used [50, 55]. IVIg recipients are more likely to attain a platelet increase within 24 h at a dose of 1 g/kg (1–2 infusions over 2 days) compared with the historical treatment regimen (0.4 g/kg/day over 5 days) [56]. The ASH guidelines suggest that, if IVIg is used, the dose should initially be 1 g/kg as a one-time dose. This dosage may be repeated if necessary [49]. A small number of patients who fail to respond to this dose may respond to a second infusion [17, 50]. If patients do not tolerate this large volume of infusion, smaller doses given over a number of days may be administered [50]. The infusion may be repeated if necessary to prevent hemorrhage and maintain platelet counts at a level safe for delivery [50].
Although generally well tolerated, IVIg causes adverse effects in approximately 5 % of patients; these include headaches, chills, myalgia, arthralgia, and back pain [17]. Headaches can be severe with acute aseptic meningitis occurring within 72 h of administration in a minority of individuals. If side-effects occur during treatment, the infusion should be slowed or stopped to alleviate symptoms. If symptoms are anticipated, the patient may take antihistamines and/or intravenous hydrocortisone to prevent a reaction. More serious adverse events such as intravascular hemolysis, renal failure, stroke, and myocardial infarction are very rare [17]. IVIg undergoes a very rigorous production process, and transmission of viruses through IVIg has not been reported since the hepatitis C transmission reported in the 1990s [17].
15.11.3 Intravenous Anti-RhD Immunoglobulin (IV Anti-D)
Although intravenous anti-RhD immunoglobulin (IV anti-D) is widely used in patients with ITP, there are few published series describing its use during pregnancy [31, 50, 57, 58]. It has been administered for ITP in pregnancy, either alone or in combination with other therapies, when an immediate rise in platelet count is required [50, 57–59]. In a small study including ten pregnant women with ITP, IV anti-D was administered during the second and the third trimesters [57]. Many of the women were taking concomitant therapy and received the drug in an attempt to increase the platelet count. In all patients, the platelet count increased above 30 × 109/L without severe side-effects [57, 58]. There were no adverse events in the neonates. In particular, none of the neonates became anemic or jaundiced even though the direct antiglobulin test (DAT) was positive in 3 of the 7 RhD (Rhesus D) positive newborns [58]. Intravenous anti-D was also administered to a woman resistant to treatment with steroids and IVIg. She achieved a platelet count of >40 × 109/L and had an uneventful vaginal delivery [60]. The platelet count remained high 1 week postpartum [60].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree