18 Thorax
Imaging Modalities
Although all existing imaging modalities have found application in the diagnosis of diseases of the chest, the chest radiograph—the oldest technique—remains the most commonly ordered initial radiologic study.1 In addition to conventional screen-film techniques, digital radiography, which captures images directly in computer format, is replacing the conventional screen-film chest radiograph. Cross-sectional imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) often is the next step in the diagnostic workup. CT is the second most commonly used thoracic imaging technique offering the most comprehensive examination of the mediastinum, lung parenchyma, pleura, and chest wall. The advent of helical CT in the 1990s allowed faster scanning and volume acquisition of data with reformation options and three-dimensional (3D) reconstruction capabilities. Helical CT was made possible by the development of slip-ring technology, which allows the tube and detector to rotate around a continuously moving table on which a patient lies. Thus, the tube and detector acquire data in a corkscrew, or helical, path. The next generation of scanners, the multidetector-row CT (MDCT) scanners, took the helical technology one step further by simultaneously acquiring data through multiple detectors. In addition to further decreasing scanning time, multidetector technology reduces or even completely eliminates the mismatch between longitudinal (axial) and transverse resolution, which further improves 3D reconstruction capabilities. MDCT decreases motion artifact and improves the quality of images, particularly in a patient in distress or an uncooperative pediatric patient. Faster scanning also allows the entire examination to be performed during optimal contrast enhancement of the cardiovascular system. For example, faster imaging using an MDCT scanner allows CT angiography for evaluation of pulmonary embolism and vascular abnormalities. Three-dimensional reconstruction allows anatomic examination of the airway and virtual bronchoscopy.
Magnetic resonance imaging (MRI) is based on the magnetic properties of the proton (the nucleus of the hydrogen atom). MRI contrast depends on several factors, including the difference in the behavior of protons in different tissues in response to magnetization as well as proton density. MRI provides soft tissue contrast resolution superior to that of CT.2 MRI contrast agents, most of which are based on gadolinium, are widely used, with a safety record superior to that of iodinated contrast agents used in CT scanning.2
MRI offers the advantages of excellent soft tissue contrast, multiplanar capability, intrinsic flow sensitivity, and lack of ionizing radiation.2 In clinical applications, MRI of the thorax is reserved for (1) specific problematic cases where mediastinal, chest wall, vertebral body, or vascular invasion needs to be further assessed after a CT of the chest has been performed, or (2) evaluating the mediastinum of patients for whom the administration of iodinated contrast material is contraindicated. The multiplanar capability of MRI aids in the evaluation of chest wall and pleural abnormalities, particularly in the apical regions.3 CT and MRI may play complementary roles in evaluating chest wall disorders such as mesenchymal tumors, primary and secondary malignancies, inflammatory conditions, and infectious disease. CT more readily demonstrates soft tissue calcification and bone destruction, whereas MRI better delineates the extent of invasive tumors, soft tissue involvement, and infiltration of bone marrow as well as vascular involvement. The ability of MRI to evaluate the lung parenchyma is limited by artifact from multiple air–soft tissue interfaces, cardiac and respiratory motion, and poor signal-to-noise ratio.2
In addition to imaging based on depiction of anatomic and morphologic structures, functional imaging is widely used in thoracic imaging. 18F-fluorodeoxyglucose (FDG) is a D-glucose analog commonly used in positron emission tomography (PET) imaging of the thorax. The fluorine incorporated in the FDG undergoes decay with emission of a positron that after an annihilation reaction with an electron produces two photons that can be detected by a camera. Because malignant tissues have high rates of metabolism, they disproportionately accumulate FDG. Increased glucose metabolism by malignant cells may allow physiologic differentiation between benign and malignant abnormalities. As inflammatory lesions also can demonstrate increased uptake of FDG, differentiation between malignancy and inflammatory lesions may be difficult. At the same time, some malignant lesions such as bronchioloalveolar carcinoma may not demonstrate uptake on PET scan. Quantification of FDG uptake by a lesion may assist in this differentiation as well as affect prognosis. The standardized uptake value (SUV) is a widely used semiquantitative method for measuring FDG uptake.4–5
FDG PET is used in examination and staging of lung cancer, differentiating benign from malignant solitary pulmonary nodules, and in diagnosis and assessment of treatment response of lymphoma.6 In the staging of lung cancer, FDG PET is useful in evaluating local disease, lymph node involvement, and distant metastatic sites. The accuracy of FDG PET in demonstrating intrathoracic metastatic nodal disease is greater than that of CT or MRI. Whole-body PET is useful in detection of unsuspected extrathoracic metastases. Conventional imaging often does not allow differentiation of tumor from posttreatment scarring. Increased FDG uptake at the sites of residual radiographic abnormalities may suggest persistent or recurrent tumor.6 In addition to its role in the staging of lung cancer, whole-body PET scanning is used in the diagnosis and staging of many other neoplasms including malignant melanoma, lymphoma, colorectal cancer, and head and neck tumors. Combining the PET scan with its sensitivity to detect lesions with a CT scan’s high special resolution may increase the diagnostic accuracy of both modalities.7,8 An integrated PET/CT scanner combines a high-resolution, high-sensitivity PET scanner with a fast multidetector CT scanner. In addition to precise matching of PET and CT images, combining the two devices in one unit allows use of the CT data to measure the differences in the absorption of the PET emission by different parts of the patient’s body (attenuation correction).
Anatomy of the Thorax
Mediastinum
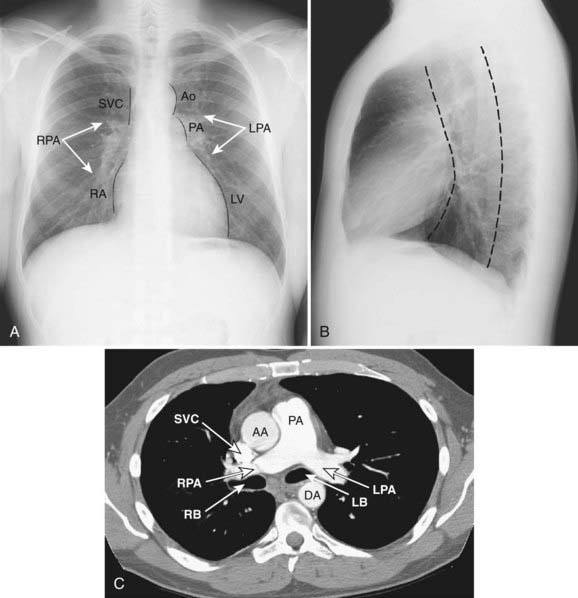
The mediastinum is commonly divided into anterior, middle, and posterior compartments based on the lateral chest radiograph. A line drawn along the back of the heart and front of the trachea divides the anterior and middle mediastinum, whereas the line connecting a point on each thoracic vertebra about a centimeter behind its anterior margin separates the middle and posterior compartments (Fig. 18-1).9,10 Neoplasms usually arise from normal structures contained in each compartment. The anterior mediastinum contains the thymus, fat, and lymph nodes, and the most common neoplasms found in that area are thymoma, lymphoma, and germ cell tumors. Thyroid abnormalities that extend from the neck into the mediastinum are most commonly found in the anterior mediastinum but may also extend to the middle and posterior mediastinum. The middle mediastinum contains the heart, pericardium, great vessels, trachea, bronchi, esophagus, and lymph nodes. Esophageal tumors, tracheal tumors, and lymph nodes are typically located in this compartment. The posterior mediastinum contains autonomic nerves, vessels, and lymph nodes. Neurogenic tumors and lymphadenopathy usually present in this location.10,11 Besides primary and secondary tumors of these compartments, other vascular and developmental lesions can occur, but they are beyond the scope of this chapter.
Diseases of the Thorax
Lung Malignancies
In 2008, there were an estimated 215,020 new cases of lung cancer in the United States: 114,690 in men and 100,330 in women. Lung cancer is the leading cause of cancer death in both men and women, accounting for 31% of all cancer deaths in men and 26% of all cancer deaths in women. Smoking is responsible for at least 30% of all cancer deaths and 87% of all lung cancers; compared to nonsmokers, lung cancer mortality rates are 22 times higher for male and 12 times higher for female smokers. In addition, an estimated 3000 nonsmoking adults die each year from lung cancer caused by secondhand smoking.12
Histologically, the most common subtypes of lung cancer, in order of decreasing frequency, include adeno, squamous cell, small cell, and large cell carcinoma. Adenocarcinomas are further subdivided into several histologic subtypes, including bronchioloalveolar carcinoma. There are major differences in therapeutic approaches to patients with small cell lung carcinoma and other types of lung cancer; therefore, all primary lung malignancies are usually divided into non–small cell and small cell categories.13,14
Non–Small Cell Lung Cancer
The most common cell types of lung cancer have a certain, typical radiographic presentation. It should be noted, however, that there is significant overlap between radiographic appearances of lung cancer. In addition, the relative frequency of different categories of this disease, as well as “typical” radiographic findings, have been changing in the past few decades.15
Adenocarcinoma
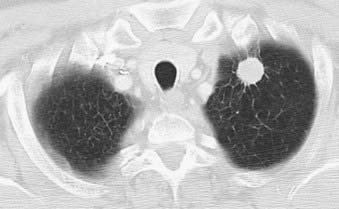
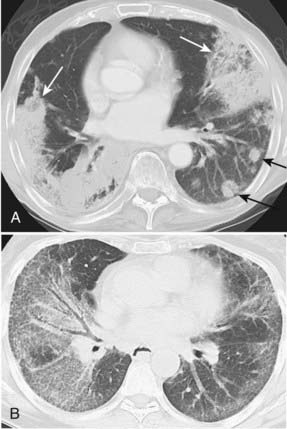
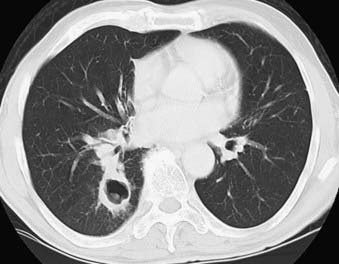
Adenocarcinoma is the most common cell type of bronchogenic carcinoma and accounts for approximately half of all cases. It typically presents as a small (often <3 cm), peripheral, round or oval solitary pulmonary nodule. It is commonly smoothly marginated, but spiculated margins can also occur (Figs. 18-2 to 18-4).14 Calcifications are rare but are occasionally seen on CT scans. Central lesions have a higher frequency of hilar and mediastinal metastases at presentation.14 Distant metastases are frequently present at the time of diagnosis. Peripheral tumors may directly invade the pleura and grow circumferentially around the lung, mimicking diffuse malignant mesothelioma.16 Central tumors may directly invade mediastinal structures or extend via the pulmonary veins to invade the left atrium.
Bronchioloalveolar Carcinoma
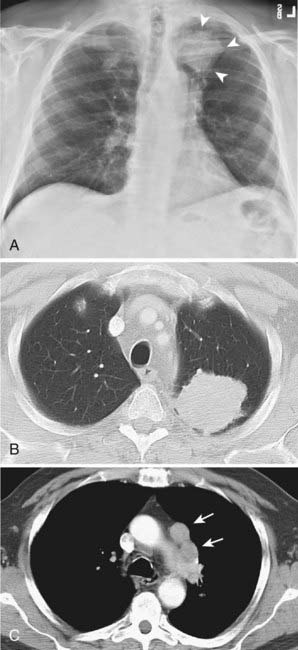
Bronchioloalveolar carcinoma, a subtype of adenocarcinoma, commonly presents as a well-circumscribed, peripherally located solitary nodule.14,17 CT scan may reveal surrounding ground glass opacity. Cavitation is an infrequent finding in adenocarcinoma.18 Bronchioloalveolar carcinoma can present as multifocal disease; several patterns have been described. It may present as multiple well-defined nodules of varying size involving one or both lungs. Another pattern of presentation can be focal, poorly defined or scattered lung opacities that may coalesce, causing opacification of a lobe or rarely the entire lung, and resemble pneumonia. Reticulonodular opacities similar in appearance to interstitial lung disease have been described (Fig. 18-5).14,18 High-resolution CT may demonstrate air attenuation and pseudocavitation within the nodules corresponding to small bronchi and cystic spaces.14,19 Unusual radiographic appearances include lobar atelectasis and expansile consolidation without air bronchograms.14,20,21 On FDG PET scans, bronchioalveolar cell carcinoma can result in a false-negative FDG scan.6,22
Squamous Cell Carcinoma
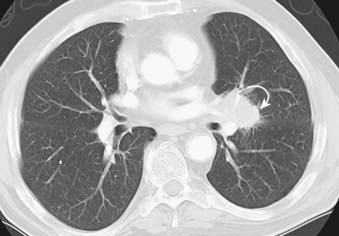
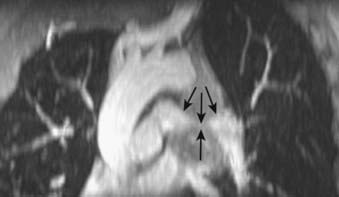
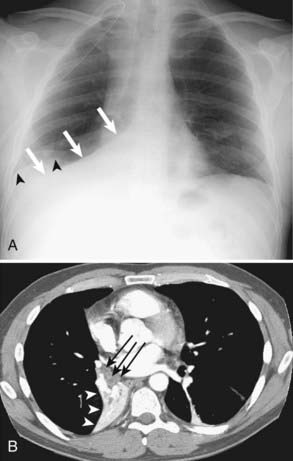
Squamous cell carcinoma is a slow-growing malignancy with late metastasis predominantly to the liver, adrenal glands, kidneys, and bones.14 Its typical presentation is described as a central endobronchial obstructing lesion with associated atelectasis or postobstructive pneumonia (Figs. 18-6 and 18-7). In addition, mucoid impaction or bronchiectasis may occur.14 With increasing frequency,15 these tumors present as a solitary peripheral nodule with or without cavitation.23 When the tumor cavitates, the inner wall is typically thick and irregular and, if secondarily infected, may develop an air-fluid level (Fig. 18-8).24 Extensive necrosis, however, may result in a thin-walled cavity. Squamous cell carcinoma is the most common histologic type found in Pancoast or superior sulcus tumors.14
Large Cell Carcinoma
The majority of large cell carcinomas present as a large (average size >4 cm) peripheral mass with poorly defined margins. Cavitation and calcification of the tumor may occur. These tumors grow rapidly and metastasize early via lymphatic and hematogenous routes, often presenting with hilar or mediastinal adenopathy.14 Giant cell carcinoma is a subtype with multiple giant cells and a more aggressive behavior and poorer prognosis.
A variant of large cell carcinoma is large cell neuroendocrine carcinoma (LCNEC), or intermediate cell neuroendocrine carcinoma. It has a poorer prognosis than classic large cell carcinoma.25 The ability to express neuroendocrine markers also places LCNEC into a broad category of neuroendocrine tumors of the lung, including atypical carcinoid, typical carcinoid, and small cell lung carcinoma.25,26
Multiple Lung Primary Tumors
Multiple lung carcinomas are present in less than 1% of all lung cancers. Synchronous lesions are defined as the simultaneous presence of tumors that are physically distinct and separate, and can have different histology. If the histology is the same, however, the tumors should be present in different segments or lobes of the lung and originate from carcinoma in situ. No carcinoma in lymphatics or extrapulmonary metastases should be present at the time of diagnosis. Simultaneous lesions not meeting these criteria most likely represent metastases.27 Metachronous lesions are defined as a second primary cancer appearing after a time interval in a patient considered cured of the initial cancer. Metachronous lesions comprise at least two thirds of multiple pulmonary neoplasms, and on average are recognized 4 to 5 years after the first primary. Up to one third of patients surviving resection for lung cancer may develop a second primary tumor.28 These lesions are regarded as metachronous primary tumors rather than metastases if they show unique histologic features. If histology is the same, then in order to meet the criteria for a metachronous lesion, the cancer-free interval should be at least 2 years and the tumor must originate from carcinoma in situ or in a different lobe or lung. There should be no carcinoma in lymphatics common to both the old and the new lesions, and no extrapulmonary metastasis should exist at the time of diagnosis.27 Squamous cell cancer is the most common histologic type of multiple carcinomas; adenocarcinoma, however, is currently the most common cell type. The reason for this trend is unknown, but the change in contents of cigarettes and filters that now contain more nitrates and less nicotine is suspected.29,30
Staging of Non–Small Cell Lung Cancer
Accurate preoperative staging in patients with non–small cell lung cancer is important for selecting those patients with localized disease who are likely to benefit from surgical resection.31,32 Cure rates and survival for non–small cell lung cancer are predicted by staging.33 The TNM staging system of the American Joint Committee on Cancer is the most widely accepted and used classification system for preoperative and postoperative staging. Mediastinal lymph nodes are involved with disease in approximately one quarter of newly diagnosed lung cancer patients.31,34 The evaluation of these lymph nodes is the primary goal of intrathoracic staging of non–small cell lung cancer. The current noninvasive imaging techniques evaluate lymph nodes based on either size (CT) or metabolism (PET).35 Conventional cross-sectional imaging techniques provide excellent anatomic information used in staging, but they have limitations differentiating benign and malignant lymph nodes based on size criteria. CT of the chest remains the most widely accepted imaging modality for evaluation of the primary tumor (the T classification); nodal metastases (the N classification); and distant metastases, especially the adrenal glands (the M classification).13
Primary Tumor Staging—T Classification
Even though it is not absolutely essential, intravenous contrast is usually administered when a CT scan of the chest is performed for staging. Intravenous contrast helps distinguish between normal vascular structures and lymph nodes and aids in evaluation of the degree of mediastinal invasion.33 PET/CT can be used in assessing the primary tumor however false-positive results can occur and may be caused by metabolically active infectious or inflammatory lesions. Granulomatous disease like sarcoidosis, tuberculosis or fungal granulomas can commonly produce significant FDG accumulation.36 Also FDG may not be useful if lesions are too small and below the threshold of PET resolution. FDG PET showed no demonstrable benefit in diagnosis and staging of non–small cell lung cancer 2 cm in size or less.37
Nodal Staging—N Classification
CT alone, however, often cannot accurately differentiate between benign lymph node enlargement and metastasis, and its accuracy for mediastinal staging has not improved over the past decade despite improvement in resolution.31 For example, normal-sized lymph nodes may contain metastases, whereas enlarged lymph nodes may have benign underlying etiology. The most commonly used criterion is a short axis diameter equal to or greater than 1 cm, even though up to 40% of lymph nodes identified as abnormal using this method can be benign. CT scanning, however, provides an extra benefit of guidance when selective biopsy is required.33 PET can be useful in suggesting metastatic involvement of lymph nodes, but it can be falsely positive in a number of inflammatory and infectious conditions. In addition, very small lesions, typically less than 1 to 1.2 cm, can be beyond the detection ability of PET. Current evidence indicates that PET is more sensitive and specific for evaluation of mediastinal lymph nodes.31,38,39 In addition, PET may be superior to CT in more accurately predicting long-term survival in non–small cell lung cancer patients.40 PET/CT is more accurate than PET alone for nodal staging of non–small cell lung cancer.41 Staging of non–small cell lung cancer is very important in guiding treatment modalities and predicting survival.42 FDG PET can identify involved nodal disease even when the nodes are normal sized on CT.43 In comparison to CT, FDG PET has been found to be more sensitive (57% versus 84%) and more specific (82% versus 89%) for detection of nodal disease in mediastinum in lung cancer.44 In nodal staging, FDG PET is cost-effective and can decrease the likelihood that a patient with mediastinal nodal metastases will undergo attempted surgery.45 Also, M and N staging with PET is superior compared to CT.46
The role of MRI currently remains limited to evaluation of superior sulcus tumors, brachial plexus, and vertebral invasion. Intravenous contrast may improve the accuracy of MRI in detection of abnormal mediastinal lymph nodes.33 MRI’s accuracy for anatomic imaging of mediastinal lymph nodes is comparable with that of CT and exceeds the accuracy of CT in evaluating hilar lymph nodes.47 PET/CT currently remains the study of choice for mediastinal staging of non–small cell lung cancer.33,47
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree