Fig. 15.1
Operating table side support is fixed to the table side rails. (A) Center knob to adjust the arm. (B) Side knob to pull out the inner rod and adjust the length. (C) Outer knob to turn the pad in either direction

Fig. 15.2
Right forearm support
Thoracolaparoscopic Esophagectomy and Three-Field Lymphadenectomy in the Prone Position (Video 15.1)
TLE–3F: Thoracoscopic Phase
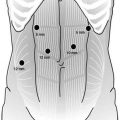
A right pneumothorax is created either by using a closed Veress needle technique or by using Visiport. Four trocars are placed into the right thoracic cavity (Fig. 15.2). The first trocar (10 mm) for the camera is placed in the fifth intercostal space corresponding to the level of the arch of the azygos vein. The second trocar (5 mm) for the work performed with the right hand is placed in the third intercostal space. The third trocar (10 mm) is placed in the seventh intercostal space. The fourth trocar (5 mm) is placed in the ninth intercostal space. The 10-mm trocar in the seventh space is useful for applying clips, vascular clamps, and taking sutures into the thorax; the camera is sometimes used in this trocar during mobilization of the lower esophagus (Fig. 15.3). Initially, the right pneumothorax at 10 mmHg partially collapses the right lung, which lies in the anterior compartment, and then the pressure is reduced to 6–8 mmHg. In addition to the pneumothorax, gravity also aids in keeping the collapsed lung in an anterior position. Two-lung ventilation is continued throughout the procedure.
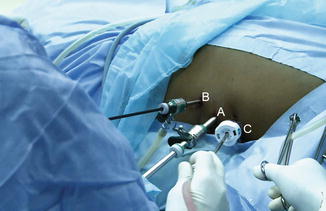

Fig. 15.3
Thoracoscopic ports. (A) Camera 10-mm port. (B) Left-hand working 5-mm port. (C) Right-hand working 5-mm port
The surgeon and camera operator stand at the patient’s right side, and the video monitor is positioned directly opposite, on the patient’s left side (Fig. 15.4). The surgeon, using a hook, incises the mediastinal pleura overlying the anterior aspect of the esophagus, and the inferior pulmonary ligament is released up to the right pulmonary vein. Anterior dissection is begun by mobilizing the esophagus away from the hilum and the pericardium [20]. Because of gravity, the heart tends to fall down anteriorly; thus, the space in front of the esophagus is widened. The mobilization extends to the level of the azygos vein, which is skeletonized and divided with double ligatures on both sides (Fig. 15.5). The posterior end of the thread is kept long and brought out through the posterior chest wall. Retraction of the thread dorsally separates the divided ends, providing a wider view. At the level of the aortic arch (Fig. 15.6), the azygos vein is the only structure that lies between the esophagus and the surgeon. Many surgeons prefer an endovascular (Endo GIA) stapler to divide the arch of the azygos vein. The parietal pleura posterior to the esophagus is incised from the level of the azygos arch vein to the crus. Blunt dissection is used to identify any potential thoracic duct branches and perforator vessels from the aorta. The thoracic duct is identified between the esophagus and aorta, and is not routinely divided. In case of tumor infiltration, the thoracic duct is clipped caudally at the hiatus and at the thoracic inlet at its insertion with the subclavian vein cranially and is transected. The esophagus is encircled with an umbilical tape, and traction by the assistant through the fourth port provides excellent exposure (Figs. 15.7 and 15.8). The surgeon is able to use both hands, simplifying the en bloc mobilization and lymphadenectomy. Initially, we used three ports, and have now changed to using four ports; the fourth port is used for traction. The operative time is shorter using the two-handed technique. The groups of lymph nodes are dissected sequentially, the subcarinal, aortobronchial, paratracheal, right recurrent laryngeal, and then left recurrent laryngeal groups (Fig. 15.9) are removed in that order [20]. The thoracoscopic approach enables removal of the cervicothoracic packet of lymph nodes along the right recurrent laryngeal nerve using a strictly “no touch” technique [16, 18].
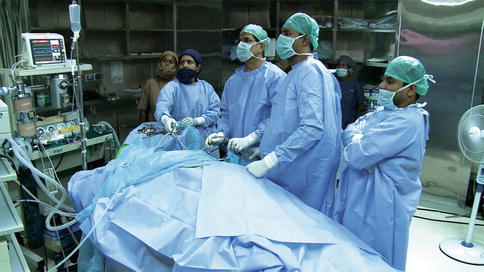
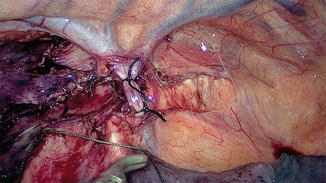
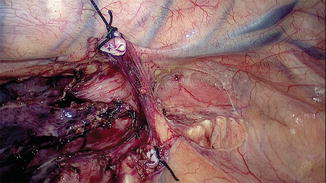
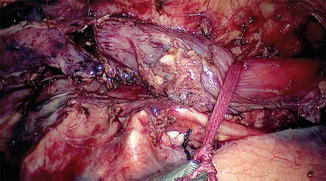
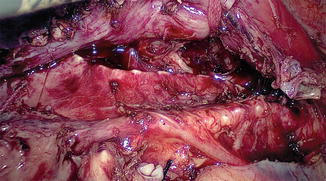
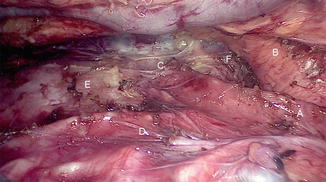

Fig. 15.4
Team setup for thoracoscopy. The patient is in the prone position, the surgeon, camera surgeon, and assisting surgeon stand on the right side of the patient and the monitor is on the left side of the patient

Fig. 15.5
After pleural incision and dissection of the azygos vein, the azygos arch is ligated doubly with silk

Fig. 15.6
The azygos arch is divided and the vertebral side is retracted for exposure

Fig. 15.7
Umbilical tape is tied around the esophagus loosely for free sliding and retraction

Fig. 15.8
Dissection of the esophagus from the trachea

Fig. 15.9
Thoracoscopic view after complete mobilization. (A) Trachea. (B) Arch of aorta. (C) Left bronchus. (D) Right bronchus. (E) Pericardium. (F) Aortopulmonary window
If tumor is present in the lower esophagus, the dissection starts from the upper chest; for upper growths, the dissection may start from the lower mediastinum. The entire periesophageal tissue and the lymph nodes are removed. If the mediastinal pleura is infiltrated, it is also excised to obtain an R0 resection. In cases of advanced tumors, where we anticipate excision of both pleura, the dissection begins from the cranial end. The esophagus may be divided by stapling and retracted laterally, which exposes the entire mediastinum. Thorough irrigation and suction of the pleural cavity is performed. A single 28-F chest tube is placed through the seventh intercostal space and the lung is re-expanded.
Abdominal/Laparoscopic Phase
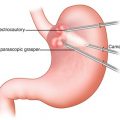
The patient is positioned supine and five ports are placed. The left lobe of the liver is retracted with instruments through the subxiphoid (epigastric) trocar. A 10-mm port for the camera is placed in the epigastrium. Two working ports, a 12-mm port in the right midclavicular line and a 5-mm port in the left midclavicular line are placed. One 5-mm port at the left anterior axillary line for gastric traction.
The lesser omentum is incised and the stomach retracted to the left and anteriorly with a grasper. The retroperitoneal lymphadenectomy (D2) is begun by incising the peritoneum at the upper border of the pancreas. The retroperitoneal lymphatic and areolar tissues are swept superiorly by skeletonizing the common hepatic artery, dissecting cranially along the lateral celiac group (Fig. 15.10). The left gastric vein is divided first at its insertion with the portal vein, followed by the left gastric artery, and then the celiac axis is completely cleared. The left gastric artery is clipped with Hem-o-lok and divided. The dissection is continued along the splenic artery up to the splenic hilum. This retroperitoneal dissection extends up to the dissected esophageal hiatus superiorly, the hilum of the spleen laterally, and the common hepatic artery and inferior vena cava medially. Finally, the lesser curvature and left gastric nodes are included with the specimen as the gastric tube is prepared.
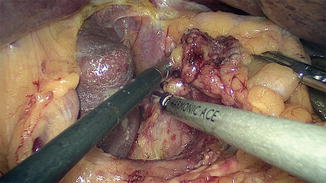

Fig. 15.10
Clearance of lymph node stations 7, 8, and 9
The right gastroepiploic artery and the arterial arcade along the greater curve is carefully assessed early to ensure its suitability as a vascular supply to the gastric conduit. The greater omentum is divided at a safe distance from the gastroepiploic arcade, and the dissection is continued upward and to the left to divide the gastrocolic and gastrosplenic ligaments by dividing the short gastric vessels, keeping the dissection closer to the origin of the left gastroepiploic artery from the splenic origin. On the right side, the dissection continues up to the second part of the duodenum. The right gastroepiploic vein is carefully protected from injury.
Gastric Tube Formation
A 5-cm-wide gastric conduit is created by means of multiple firings of an Endo GIA 6-cm cartridge (Echelon–Ethicon) through the right mid-clavicle port. The stomach is stretched when stapling starts on the lesser curvature, 5 cm away from the pylorus, and progressing toward the fundus of the stomach. A golden cartridge is used to staple the antrum and blue cartridges are used for dividing the body of the stomach (Fig. 15.11).
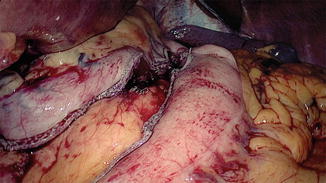

Fig. 15.11
Intracorporeal formation of the gastric tube using an Endo GIA stapler
A pyloroplasty or pyloromyotomy is performed by incising the pylorus longitudinally and the closure is performed transversely with interrupted sutures using 3-0 PDS suture. The placement of the feeding jejunostomy is at the discretion of the surgeon. Our preference is to place a nasojejunal feeding tube. Only in selected cases in which the patient develops a leak do we perform a feeding jejunostomy. The incidence of developing a leak is very low [13] and feeding jejunostomy is not without morbidity.
Cervical Phase
Specimen Extraction and Gastric Pull Up
The cervical esophagus is dissected through a left collar incision and divided. The distal end of the esophagus in the neck is over sewn and attached to a long Ryles tube. A long plastic sleeve is used as a protective sheath and attached to the esophagus with a separate stitch (Fig. 15.12). The pneumoperitoneum is reestablished and the esophagus is pulled down until the protective sheath reaches the peritoneal cavity. The stitch is released and the lower end of the plastic sheath opened (Fig. 15.13). The Ryles tube in the neck is used to pull the esophagus into the plastic sheath by the assistant; the surgeon working on the anterior wall of the gastric tube pushes the tube carefully, using a hand-over-hand technique and avoiding twisting or spiraling (Fig. 15.14).
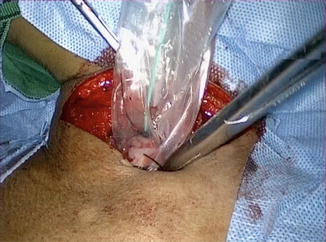
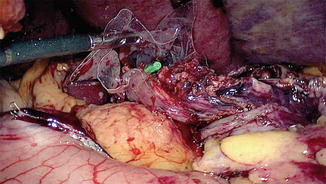
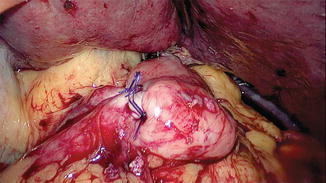

Fig. 15.12
Divided esophagus, Ryle’s tube with a covering plastic sleeve is being pushed into the posterior mediastinum

Fig. 15.13
Plastic sleeve in position (from neck wound connecting the peritoneal cavity) lying in the posterior mediastinum. (A) Nasogastric tube attached to the divided end of the cervical esophagus

Fig. 15.14
Position of the stomach tube. The pyloroplasty wound is visible
Esophagogastric Anastomosis
A small vertical gastrotomy is performed with electrocautery. The posterior wall of the esophagus and the anterior wall of the stomach are then aligned. A 3-cm long, 3.5-mm Endo GIA stapler is used to perform the posterior anastomosis. The anterior anastomosis is performed transversely using two staplers according to the modified Collard [19] or Orringer technique [21] and a wide stoma is obtained. The anterior wall may also be approximated with single-layer hand-sewn continuous suturing using a 3-0 monofilament absorbable suture, beginning at each corner and tied in the middle. After completion of the anastomosis, any redundant stomach is retracted into the abdomen. A nasogastric tube is passed carefully until it reaches the antrum of the stomach and its tip is kept above the pylorus. The gastric tube is then secured to the diaphragmatic hiatus anteriorly and laterally using long 2-0 nonabsorbable sutures to prevent intrathoracic herniation of the abdominal viscera. A nasojejunal tube is placed across the pylorus into the jejunum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree