Fig. 19.1
Bronchoscopic removal of aspirated foreign body
Complications associated with bronchoscopic foreign body removal occur in less than 10 % of patients and include bronchospasm, laryngospasm, pneumonia, pneumothorax, pneumomediastinum, and bleeding. It has an overall success rate of greater than 85 %. Failure of bronchoscopic removal generally requires tracheostomy or thoracotomy for object removal. Surprisingly, location or duration of time in the airway does not seem to predict complications or success [5, 6].
Massive Hemoptysis
Hemoptysis may be a common complaint among geriatric patients presenting to the emergency room, but “massive” hemoptysis will frequently prompt acute care surgery consultation. The amount of blood loss to be considered massive is variable, but generally greater than 600 ml/24 h. The actual amount may not be important, however, as significant oxygenation and ventilation deficiencies can occur with a much smaller volume of blood. Most patients presenting with massive hemoptysis will develop life-threatening respiratory failure before hemodynamically significant blood loss occurs [7]. This is of particular importance to the geriatric population who may already have diminished pulmonary reserve.
Ninety percent of the bleeding in cases of massive hemoptysis originates from the bronchial arteries. Only 5 % of hemoptysis occurs from pulmonary arterial sources and is generally less threatening secondary to the lower pressures. The specific causes of massive hemoptysis are broad, but certain etiologies are more common among the geriatric population. Neoplastic etiologies including both primary and metastatic lesions are the most common. Other causes include infections such as bronchiectasis, tuberculosis, fungal balls, lung abscess, and necrotizing pneumonia. Vascular etiologies are less common, but may still be seen and include AV malformations, thoracic aneurysm, pulmonary embolism/infarction, and mitral stenosis. Medical causes such as Goodpasture syndrome and Behcet’s disease are also causes, but are rare among the elderly [7].
Clinical Presentation and Diagnosis
Patients presenting with massive hemoptysis require a prompt assessment that should mirror that of a trauma resuscitation (Fig. 19.2). The airway must be assessed first. Patients with severe bleeding or signs of respiratory failure should be intubated without delay. The largest possible endotracheal tube should be used to facilitate suctioning as well as bronchoscopy. Physical exam may reveal the side of bleeding in some patients. If this is the case, patients should be placed in the lateral decubitus position toward the side of bleeding in order to diminish the risk of aspirating blood into the contralateral lung. Large-bore intravenous access should be in place and fluid resuscitation should commence. Patients with unstable vital signs should be transfused packed red blood cells [7].
The diagnostic evaluation of massive hemoptysis is secondary to the initial stabilization and resuscitation, but can generally proceed in tandem. A sputum examination is recommended for all patients to look for the presence of bacteria. Cultures should be obtained, especially looking for mycobacterium and fungus. Chest x-ray is also a useful initial diagnostic tool. Parenchymal pathologies such as tumors, cavitary lesions, and infiltrates may be readily apparent and help localize the portion of lung responsible for bleeding. It is important to note, however, that greater than 20 % of patients with massive hemoptysis may have a normal chest x-ray. Computed tomography (CT) is also an important tool for evaluation. It may demonstrate small lesions such as tumors or bronchiectasis not readily seen on plain films. When performed with intravenous contrast, it is the preferred method to diagnose thoracic aneurysm or other vascular abnormalities. Caution should be exercised in obtaining CT scans on patients with unstable vital signs or unsecured airways [7, 8].
Bronchoscopy is usually the most effective method for bleeding localization. Both rigid and flexible bronchoscopy can be performed. Rigid bronchoscopy has the benefit of greater suctioning ability and maintenance of airway patency in cases of heavy bleeding, but cannot be used to access peripheral lesions. It also requires general anesthesia in all but the most experienced hands. Flexible bronchoscopy can be performed at the bedside and may easily reach the distal bronchioles, but visualization of heavily bleeding lesions may prove challenging. Occasionally, the installation of dilute epinephrine or other vasoactive agents may reduce bleeding and improve visualization [7, 8].
Therapeutic Options
Therapeutic options for massive hemoptysis are broad and vary depending upon the etiology. Surgical intervention was traditionally the method of choice, but other less invasive options can be effective. In some cases, definitive surgical therapy may be delayed following temporary control of bleeding to allow for preoperative optimization.
Endobronchial methods are commonly first-line therapies, as they can be instituted at the time of bronchoscopic localization. Techniques include the installation of local hemostatic agents, photocoagulation, and endobronchial tamponade using balloon catheters. Endobronchial methods are most often utilized as temporary measures to allow for resuscitation and planning of more definitive intervention, but this depends upon the etiology [7, 8].
Bronchial artery embolization is a highly effective technique with a greater than 90 % success rate at 24 h. Selective angiography is used to identify the bleeding bronchial artery, followed by the instillation of coils or other thrombotic particles. Failure of this technique may occur secondary to non-bronchial collateral circulation [7, 9].
Patients with massive hemoptysis secondary to aspergilloma may be treated with the direct installation of antifungal drugs into the bleeding cavity. This can be accomplished via either percutaneous or transbronchial catheter access. This minimally invasive technique may be particularly useful for patients who are poor surgical candidates. External beam radiation therapy has also been used with success in hemoptysis from fungal balls, but only for those with low rates of bleeding [7].
Surgical intervention includes the full spectrum of pulmonary resection, including pneumonectomy. Patients who have a localized source of bleeding that would be amenable to complete resection and who are otherwise appropriate candidates for surgery should be considered. It is the treatment of choice for patients with malignancies or cavitary diseases in which vascular control alone would not be curative. Surgery is contraindicated in patients with lung carcinoma invading the mediastinum, trachea, heart, great vessels, or parietal pleura. Options must be carefully considered among patients with significant preexisting lung dysfunction, as emergent lobectomy or pneumonectomy will be poorly tolerated with diminished pulmonary reserve [7–9].
Outcome
The mortality rate for patients with massive hemoptysis is difficult to quantify and depends heavily on the underlying etiology. Patients with malignancy or significant bleeding (>1,000 ml/24 h) tend to do worse than those with other presentations. There is no evidence to suggest that age in and of itself is a risk factor for poor outcome [7].
Lung Abscess
Lung abscess is a well-circumscribed collection of pus within the lung that leads to cavity formation and the presence of an air-fluid level on imaging studies. It is most commonly formed by anaerobic bacteria and follows aspiration. Other etiologies include necrotizing pneumonia, septic emboli, and prior cavitation. Patients with a predisposition to aspiration, underlying immunocompromised state, or bronchial obstruction are at increased risk. Advanced age in and of itself is not a risk factor for lung abscess development, but has been associated with poor outcome when present [10–13].
Clinical Presentation and Diagnosis
Lung abscess typically presents with cough, pleuritic chest pain, fevers, weight loss, hemoptysis, or dyspnea. Patients will typically have one or more underlying risk factors by history and usually describe a prolonged course of symptoms prior to presentation. Diagnosis is usually straightforward and can be accomplished with plain chest radiography or computed tomography.
Management
The majority of lung abscess cavities will communicate with the bronchial tree and drain spontaneously; thus, antibiotic therapy alone is the primary treatment (Fig. 19.1). Antibiotics are usually chosen empirically because accurate culture data from within the abscess cannot be obtained noninvasively. There is an increasing incidence of gram-negative pathogens among community-acquired organisms. Current regimens use a β-lactam with a β-lactamase inhibitor or combination therapy with an advanced-generation cephalosporin and clindamycin or metronidazole. Unusual organisms such as gram-positive aerobes are rarely found to be the etiology of lung abscess and are more common in nosocomial cases [10].
Prolonged antibiotic therapy is successful in greater than 85 % of cases. Unfortunately, the geriatric population is at increased risk for conservative treatment failure. Other risk factors for failure include immunocompromised states, large abscess cavities, and unresolved bronchial obstruction. Antibiotic treatment failure requires improved drainage of the abscess. This can usually be accomplished percutaneously with radiographic guidance and represents definitive therapy for the majority of patients. A small minority of patients will require surgery for clearance. Surgical intervention for lung abscess includes varying degrees of pulmonary resection, including complete pneumonectomy in rare cases. Both traditional and minimally invasive techniques can be utilized. As surgery is taking place in an infected field, there is an increased risk of bronchial stump breakdown and bronchopleural fistula. Tissue coverage over the bronchial stump is recommended if possible [10, 12].
Outcome
Empyema
Empyema refers to the presence of infected fluid within the pleural space. The most common etiology is infection arising in the ipsilateral lung from pneumonia, lung abscess, or bronchiectasis. Other causes include trauma, postsurgical, esophageal perforation, and spread of intra-abdominal infection across the diaphragm. Primary empyema may rarely occur and is most often due to hematogenous spread of bacteria from gingival and upper respiratory tract infections or tuberculosis. Empyema is seen in about 60,000 patients annually and is of particular importance to the geriatric population because it is more common at the extremes of age [11, 14].
Clinical Presentation and Diagnosis
Patients with empyema typically present with pleuritic chest pain, fever, and nonspecific symptoms such as malaise (Fig. 19.2). Chest radiographs are commonly the first-line diagnostic study and can demonstrate pleural effusion and sometimes loculations, but cannot confirm bacterial infection. Similarly, ultrasound can easily demonstrate pleural fluid collections, but does not confirm empyema. Computed tomography is the most useful diagnostic tool. It can demonstrate and quantify pleural fluid collections, loculations, and pleural enhancement or thickening. Though CT findings can be highly suggestive, definitive diagnosis requires aspiration of purulent fluid or gram-stain/culture results [11, 14] (Fig. 19.3).
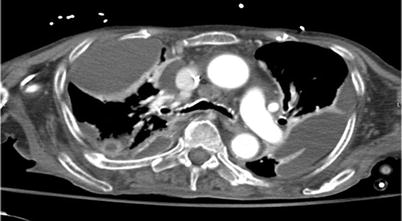

Fig. 19.3
CT scan demonstrating loculated fluid collections suggestive of empyema
Management
The treatment of empyema involves drainage of infected fluid with lung re-expansion and antibiotic therapy. The choice of drainage method depends on the phase of the underlying empyema: exudative, fibrinopurulent, or organized. The first two phases can often be managed successfully without surgery [14].
The exudative phase of empyema is characterized by free-flowing fluid with minimal to no loculations. Tube thoracostomy alone is generally sufficient to achieve complete drainage of the empyema. In the fibrinopurulent phase, the fluid is thicker and loculations are common. Sufficient drainage by thoracostomy tube is difficult and may require multiple drains in specific locations. The bedside installation of lytic agents, particularly streptokinase, has been shown to be an effective method to assist the drainage of loculated collections. In many patients, drainage tubes may be required for a prolonged amount of time to prevent reaccumulation. Closed suction drains can be converted to open drains once the lung becomes adherent to the chest wall and gradually backed out over time. If adequate drainage of an early-phase empyema is unsuccessful by percutaneous methods, surgical intervention should be considered [10, 14, 15].
The organized phase of empyema occurs over a period of 4–6 weeks and is characterized by the development of a fibrous “peel” of visceral pleura that prevents lung expansion. Surgical intervention is required to decorticate the lung and remove all infected material. Access to the pleural space can be achieved using VATS or traditional thoracotomy, but minimally invasive approaches are less successful in more advanced cases. Incomplete decortication will not allow full expansion of the lung and obliteration of the infected cavity; thus, reaccumulation will occur. In rare cases in which the pleural cavity cannot be obliterated, an open drainage procedure or muscle rotational flap may be necessary to control the empyema. Patients who cannot tolerate thoracotomy and proper decortication may also be candidates for an open drainage procedure under local anesthetic (assuming the presence of a mature empyema and adherent lung) [10, 11, 14].
Spontaneous Pneumothorax
Spontaneous pneumothorax is classified as either primary or secondary, depending upon the presence of underlying lung disease. Primary pneumothorax generally occurs in tall, thin persons between the ages of 10 and 30 years and rarely occurs in individuals over the age of 40. Secondary spontaneous pneumothorax (SSP), however, has a peak incidence between 60 and 65 years of age and is frequently seen among the geriatric population (Fig. 19.4). It is of particular importance because it can frequently be life-threatening, largely due to the underlying lung disease and low cardiopulmonary reserve. The most common cause of SSP among the geriatric population is underlying chronic obstructive pulmonary disease (COPD), representing greater than 70 % of cases. The probability of pneumothorax increases as the severity of COPD worsens. Other etiologies of SSP in the geriatric population include pulmonary fibrosis, autoimmune diseases, and infectious etiologies [16, 17].
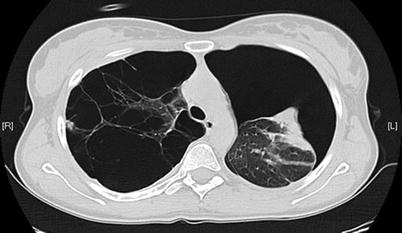

Fig. 19.4
Spontaneous pneumothorax in the presence of significant bleb disease
Clinical Presentation and Diagnosis
Patients with SSP will present with dyspnea and frequently unilateral chest pain. Severe hypoxemia or hypotension can occur, even with smaller-sized pneumothoraces. Hypercapnia is also extremely common. The diagnosis should always be suspected among patients with known underlying COPD and new onset dyspnea or chest pain. A high index of suspicion is essential, as rapid diagnosis and treatment may be lifesaving [16].
The diagnosis of SSP is usually made with chest x-ray, but can be challenging in patients with large bullous lesions. Radiographically, a pneumothorax should appear as a visceral pleural line that runs parallel to the chest wall. Large bullous lesions that abut the chest wall generally have a concave appearance. If the diagnosis is unclear, computed tomography of the chest can be used for differentiation [16].
Management
Patients with SSP should be treated urgently with chest tube placement with water seal drainage. The chest tube apparatus can be placed to suction for patients with large air leaks or incomplete re-expansion of the lung. A smaller-bore tube (28 french or less) is generally sufficient. Chest tube drainage alone will resolve most SSP, but may require a prolonged time course if there is persistent air leak [16, 18].
Most surgeons would recommend intervention following the first episode of SSP in order to prevent recurrence, as this could be rapidly life-threatening. The least invasive treatment is the installation of sclerosing agents through the chest tube, but this also carries the highest rate of recurrence (up to 25 %). It is even less effective in the presence of an air leak, which is common among SSP. Some patients with SSP may be poor overall surgical candidates, particularly among the geriatric population. In these patients, bedside chemical pleurodesis may be the only viable option to prevent recurrence [16, 18].
Surgical intervention for SSP involves resection of the ruptured bleb and surrounding bullous disease in conjunction with mechanical or chemical pleurodesis. Options for the geriatric patient include standard posterolateral thoracotomy, limited thoracotomy, median sternotomy, and video-assisted thoracoscopic surgery (VATS) approaches. Each approach carries specific advantages and disadvantages, and the decision should be made on an individual basis. Thoracotomy offers the best exposure and has the lowest recurrence rate, but is associated with significant postoperative pain and morbidity. VATS may be less invasive, but has a higher recurrence rate among published series. In addition, VATS generally requires single-lung ventilation of the contralateral lung to allow visualization of the affected lung. Many patients with SSP will not be able to tolerate single-lung ventilation secondary to their underlying pulmonary disease [16–19].
Outcome
The outcome for patients with SSP depends largely on the severity of the underlying lung disease and comorbidities. Overall mortality rates are generally low (<6 %) among geriatric patients with a COPD etiology. Patients with recurrence or who require invasive operative intervention may have prolonged hospital stays and significant morbidity, however.
Aorta
Acute aortic pathology is an important consideration among the elderly, as the major disease processes have an increasing incidence with age. The conditions discussed generally require subspecialized care for definitive management, but initial diagnosis and stabilization may be frequently required of the acute care surgeon.
Acute Aortic Syndrome
Acute aortic syndrome (AAS) is a term used to describe a group of conditions with the common and primary presenting symptom of aortic pain. The conditions that comprise AAS include acute aortic dissection, penetrating aortic ulcer, and intramural hematoma of the aorta. Thoracic aortic aneurysm is a distinct entity, but acute presentations (rupture, expansion, etc.) behave similarly in terms of symptoms and diagnostic workup.
Clinical Presentation and Diagnosis
Patients with AAS typically present with pain that is usually of sudden onset and with maximal initial intensity. It is generally in the substernal area and is classically described as having a “sharp,” “tearing,” or “ripping” character. The most sensitive description of the pain appears to be the abruptness of onset, as this is present in over 90 % of cases. A careful and thoughtful history is of paramount importance, as up to 30 % of patients ultimately found to have AAS will be initially misdiagnosed [20, 21].
The currently available diagnostic modalities for AAS include computed tomography (CT), transesophageal echocardiography (TEE), magnetic resonance imaging (MRI), and aortic angiography. Contrast-enhanced CT is the most commonly employed as it is readily available and noninvasive. Chest x-ray (CXR) can be suggestive of AAS in up to 85 % of cases, showing a widened mediastinum, pleural effusion, or subtle cardiac contour changes. Electrocardiogram (EKG) tracings in AAS may be abnormal, but are generally nonspecific [20, 21].
AAS is an important consideration among the elderly population because it is predominantly a disease of older people, with at least a third occurring over the age of 70. It is more common among males and is associated with the common comorbidities for atherosclerotic disease such as hypertension and smoking [20, 21].
Acute Aortic Dissection (AAD)
AAD arises from a tear within the intima of the aorta, leading to a flow of blood within the media layer of the aorta. A second or “false” lumen is created as a consequence and can rapidly propagate secondary to the high underlying pressures within the aorta. The diversion of blood flow within the false lumen can lead to ischemia or thrombosis of involved aortic branches. A careful pulse exam is an essential component of the early evaluation, as it may reveal selective deficits that suggest AAD. Dissections of the ascending aorta can have involvement of the coronary ostia and produce cardiac ischemia with associated electrocardiogram changes and enzyme elevations. Consideration of AAD among patients with the appropriate history is important, as confusion with acute coronary syndromes may lead to the premature and potentially disastrous administration of anticoagulation or thrombolytics. Approximately 5 % of AAD is iatrogenic following open cardiac procedures or catheter-based interventions. The iatrogenic AAD population tends to be older and with a higher incidence of underlying vascular disease [20, 22, 23].
Management
The mainstay of medical therapy for AAD is immediate blood pressure control if hypertension is present to try to limit propagation of the dissection. A combination of B-blockers and vasodilators should be employed to reduce the force of ventricular contraction. The goal for systolic blood pressure should be less than 120 mmHg, as long as cerebral and end-organ perfusion is maintained. Surgical interventions vary depending on the type of dissection present. There have been several classification schemes used to describe AAS, but among the most common is the Sanford classification. It describes AAD in terms of its involvement with the ascending aorta (type A) (Fig. 19.5) or the descending aorta (type B) (Fig. 19.6). This division is important, because type A dissections are considered true surgical emergencies. The mortality rate for type A dissections increases significantly with delays to surgical intervention. Factors contributing to mortality include rupture into the pericardium leading to tamponade, coronary vessel involvement and myocardial ischemia, distal organ malperfusion, rupture into the pleural space, or valvular involvement leading to acute cardiac failure [20, 21].
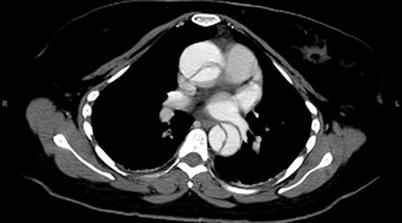

Fig. 19.5




Type A (ascending arch) aortic dissection [23]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree