Although germ cell tumors (GCT) are among the most curable solid tumors, a subset of patients with GCT experience relapse or progression despite appropriate cisplatin-based therapy or first-line salvage therapy. This article describes the molecular mechanisms of cisplatin resistance, outlines single-agent chemotherapy and combination chemotherapy regimens that are active against GCT in the third-line or later setting, discusses the use of drug therapy for treating growing teratoma syndrome and teratoma with malignant transformation, outlines novel agents used to treat GCT, and highlights ongoing clinical trials and future directions in the treatment of refractory GCT.
Germ cell tumors (GCT) are one of the most curable solid tumors, with a cure-rate of 70% to 80% for patients with metastatic disease who are treated with cisplatin-based combination chemotherapy. Prognosis of patients who do not respond adequately to first-line therapy or who relapse after first-line treatment is poor, with a 20% to 25% cure-rate for those treated with second-line cisplatin-based chemotherapy. High-dose chemotherapy plus autologous peripheral blood stem cell transplant results in durable responses in 30% to 50% of patients with relapsed disease. Unfortunately, a subset of patients experience relapse or progression despite salvage chemotherapy, including those with cisplatin-refractory (response or disease stabilization during chemotherapy but disease progression within 4 weeks after cisplatin-based chemotherapy) and those with absolutely cisplatin-refractory disease (progression during cisplatin-based treatment). These patients have a very poor prognosis. This review focuses treatment options for patients with multiply-relapsed or cisplatin-refractory disease, and describes the molecular mechanisms of cisplatin resistance, outlines single-agent chemotherapy and combination chemotherapy regimens that are active against GCT in the third-line setting, discusses the use of chemotherapy for the growing teratoma syndrome, outlines novel agents that have been used in to treat GCT, and highlights ongoing clinical trials and future directions in the treatment of refractory GCT.
Molecular mechanisms of cisplatin resistance
Although GCTs generally display exquisite sensitivity to cisplatin, resistance occurs in approximately 20% of patients with metastatic GCT. Resistance to cisplatin can be intrinsic or acquired after repeated exposure to the drug. The molecular mechanisms of cisplatin resistance in GCT arise from a complex series of events that are not yet completely understood. Cisplatin induces apoptosis in sensitive cells by first gaining entry into the cell, then binding with DNA to form DNA adducts. These adducts distort DNA, which is then recognized as damaged, activating the apoptotic cascade. Molecular alterations leading to cisplatin resistance can occur before cisplatin-induced DNA damage occurs, during the process of DNA damage and repair, or after cisplatin-induced DNA damage has occurred ( Fig. 1 ). Multiple molecular alterations may exist at various points in the process, leading to cisplatin resistance.
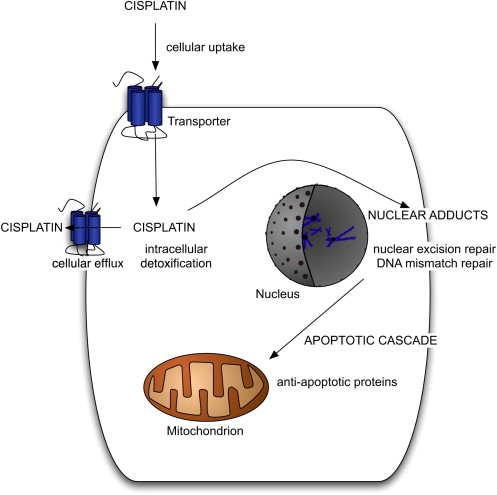
Before the occurrence of cisplatin-induced DNA damage, decreased cellular uptake of cisplatin, increased cellular efflux of the drug, or intracellular detoxification of the drug could all potentially lead to resistance. A copper transporter may be involved in the cellular uptake of cisplatin, although little is known about this transporter or its potential for implication in cisplatin resistance. Several drug transporters, including the multidrug resistance–associated proteins in the ATP-binding cassette (ABC) transporter family and lung resistance–related protein, have been suggested to transport cisplatin out of cells, possibly leading to cisplatin resistance. Studies examining the role of intracellular detoxification of cisplatin by glutathione or metallothionein have had mixed results, with one study suggesting a positive correlation between levels of these thiols and cisplatin resistance in testis tumor cell lines, and a second study finding no correlation between glutathione content and cisplatin resistance in a GCT line or a colon cancer cell line.
Once DNA adducts form, mechanisms related to the repair or removal of damaged DNA may contribute to cisplatin resistance. Nuclear excision repair (NER) is a major pathway for the repair of DNA damaged by cisplatin. GCT cell lines have been shown to have a low intrinsic capacity for nuclear excision repair, perhaps related to low levels of xeroderma pigmentosum group A protein (XPA) or the ERCC1-XPF–specific endonuclease complex. Testis tumor cell lines with low levels of XPA lose their capacity to repair DNA damage, and subsequently regain full NER capacity with the addition of XPA. The reduced NER capacity of GCT might contribute to cisplatin-sensitivity, and perhaps resistant GCT have higher levels of XPA and a more robust NER pathway. However, one study of XPA levels in human samples found no differences between sensitive and resistant tumors and no correlation between XPA protein level and cisplatin sensitivity in three GCT cell lines.
Other factors involved in DNA repair include high mobility group (HMG) proteins, which have been implicated in cisplatin resistance for their role in recognizing cisplatin–DNA adducts and blocking the NER pathway from repairing the damaged DNA. A defective DNA mismatch repair system could lead to failure to recognize DNA damage and subsequent failure to initiate apoptosis. A study of 100 unselected GCT and 11 chemotherapy-resistant GCT found a significantly higher incidence of microsatellite instability (MSI), a marker of defective mismatch repair, in the resistant tumors. Furthermore, the resistant tumors exhibited MSI in several loci. A subsequent study using the same control cohort and 35 cisplatin-resistant GCT found a higher incidence of MSI in the resistant tumors, again in multiple loci. A higher incidence of BRAF V600E mutation was also seen in the resistant tumors compared with controls, and presence of the BRAF mutation was highly correlated with MSI. Although experts had previously postulated that a high level of wild-type p53 in GCT is responsible for cisplatin sensitivity, studies have shown that a high level of p53 does not correlate to chemotherapy sensitivity in GCT, that the inactivation of p53 in a cisplatin-sensitive cell line does not confer resistance, and that the inactivation of p53 is not a common occurrence in resistant GCT.
Once the apoptotic cascade has been initiated, mechanisms that inhibit apoptosis could lead to cisplatin resistance. Antiapoptotic proteins such as BAX and BCL-2 have been examined as potential mediators of cisplatin resistance in GCT, but no significant correlation with resistance has emerged. Cisplatin resistance in GCT is likely a multifactorial phenomenon, potentially involving elements of each of the molecular pathways described. Further research, particularly using human tumor samples and clinical correlation, is needed to further clarify this complex issue.
Active single agents in refractory GCTs
For patients with cisplatin-refractory GCT, additional active agents are used in the third-line or later setting ( Table 1 ). Historically, etoposide was the first agent to exhibit activity in GCTs refractory to cisplatin-based chemotherapy. Subsequently, etoposide was incorporated into the first-line combination regimen of bleomycin, etoposide, and cisplatin (BEP). A phase II study of daily oral etoposide in patients with refractory GCT, all of whom had undergone prior etoposide therapy, showed a 14% response rate.
| Drug | Author | Number of Patients | Responses | Overall Response Rate (%) |
|---|---|---|---|---|
| Etoposide | Miller and Einhorn, 1990 | 21 | 6 PR, 0 CR | 14 |
| Ifosfamide | Wheeler et al, 1986 | 30 | 6 PR, 1 CR | 23 |
| Paclitaxel | Motzer et al, 1994 | 31 | 5 PR, 3 CR | 26 |
| Bokemeyer et al, 1994 | 10 | 3 PR, 0 CR | 30 | |
| Bokemeyer et al, 1996 | 24 | 4 PR, 2 CR | 25 | |
| Nazario et al, 1995 | 15 | 2 PR, 0 CR | 13 | |
| Sandler et al, 1998 | 18 | 2 PR, 0 CR | 11 | |
| Gemcitabine | Bokemeyer et al, 1999 | 31 | 6 PR, 0 CR | 19 |
| Einhorn et al, 1999 | 20 | 2 PR, 1 CR | 15 | |
| Oxaliplatin | Kollmannsberger et al, 2002 | 32 | 4 PR, 0 CR | 13 |
| Fizazi et al, 2004 | 8 | 3 PR, 0 CR | 37 |
Ifosfamide was first shown to have single-agent activity against refractory GCT in the mid-1980s, with a response rate of approximately 20%. Currently, most patients receive ifosfamide as salvage therapy after failure to cure with first-lines regimens such as BEP, or as a component of first-line therapy in poor-risk advanced disease when bleomycin toxicity is a concern.
Paclitaxel showed activity against GCT in patients for whom cisplatin-based chemotherapy failed. Among 31 patients treated, 8 (26%) experienced a major response. None of the patients in the study who had received prior treatment with high-dose chemotherapy experienced a response to paclitaxel. The German Testicular Cancer Study Group reported that 6 of 24 patients with relapsed, mostly cisplatin-refractory GCT (25%) experienced responded to paclitaxel, including 2 who had prior high-dose chemotherapy with stem cell support, although 1 of these patients relapsed again 3 months after treatment with paclitaxel. Including these two trials, five trials of single-agent paclitaxel in cisplatin-refractory GCT have been published. These trials include a total of 98 patients, with a combined response rate of 21%.
A single case report exists of the related agent, docetaxel, leading to a durable complete remission when used as a single-agent in the third-line setting in a patient with mediastinal GCT refractory to salvage chemotherapy.
Single-agent gemcitabine has been shown to exhibit activity against refractory GCT in two phase II studies, with response rates of 19% and 15%, respectively. Both studies reported responses in heavily pretreated patients.
In vitro studies of oxaliplatin have shown its activity in cisplatin-resistant nonseminomatous GCT cell lines. A phase II study of single-agent oxaliplatin in patients with heavily pretreated refractory GCT reported a response rate of 13%. A French series of eight patients with refractory GCT treated with single-agent oxaliplatin reported a response rate of 37%, although how heavily pretreated these patients were is unclear.
Active single agents in refractory GCTs
For patients with cisplatin-refractory GCT, additional active agents are used in the third-line or later setting ( Table 1 ). Historically, etoposide was the first agent to exhibit activity in GCTs refractory to cisplatin-based chemotherapy. Subsequently, etoposide was incorporated into the first-line combination regimen of bleomycin, etoposide, and cisplatin (BEP). A phase II study of daily oral etoposide in patients with refractory GCT, all of whom had undergone prior etoposide therapy, showed a 14% response rate.
| Drug | Author | Number of Patients | Responses | Overall Response Rate (%) |
|---|---|---|---|---|
| Etoposide | Miller and Einhorn, 1990 | 21 | 6 PR, 0 CR | 14 |
| Ifosfamide | Wheeler et al, 1986 | 30 | 6 PR, 1 CR | 23 |
| Paclitaxel | Motzer et al, 1994 | 31 | 5 PR, 3 CR | 26 |
| Bokemeyer et al, 1994 | 10 | 3 PR, 0 CR | 30 | |
| Bokemeyer et al, 1996 | 24 | 4 PR, 2 CR | 25 | |
| Nazario et al, 1995 | 15 | 2 PR, 0 CR | 13 | |
| Sandler et al, 1998 | 18 | 2 PR, 0 CR | 11 | |
| Gemcitabine | Bokemeyer et al, 1999 | 31 | 6 PR, 0 CR | 19 |
| Einhorn et al, 1999 | 20 | 2 PR, 1 CR | 15 | |
| Oxaliplatin | Kollmannsberger et al, 2002 | 32 | 4 PR, 0 CR | 13 |
| Fizazi et al, 2004 | 8 | 3 PR, 0 CR | 37 |
Ifosfamide was first shown to have single-agent activity against refractory GCT in the mid-1980s, with a response rate of approximately 20%. Currently, most patients receive ifosfamide as salvage therapy after failure to cure with first-lines regimens such as BEP, or as a component of first-line therapy in poor-risk advanced disease when bleomycin toxicity is a concern.
Paclitaxel showed activity against GCT in patients for whom cisplatin-based chemotherapy failed. Among 31 patients treated, 8 (26%) experienced a major response. None of the patients in the study who had received prior treatment with high-dose chemotherapy experienced a response to paclitaxel. The German Testicular Cancer Study Group reported that 6 of 24 patients with relapsed, mostly cisplatin-refractory GCT (25%) experienced responded to paclitaxel, including 2 who had prior high-dose chemotherapy with stem cell support, although 1 of these patients relapsed again 3 months after treatment with paclitaxel. Including these two trials, five trials of single-agent paclitaxel in cisplatin-refractory GCT have been published. These trials include a total of 98 patients, with a combined response rate of 21%.
A single case report exists of the related agent, docetaxel, leading to a durable complete remission when used as a single-agent in the third-line setting in a patient with mediastinal GCT refractory to salvage chemotherapy.
Single-agent gemcitabine has been shown to exhibit activity against refractory GCT in two phase II studies, with response rates of 19% and 15%, respectively. Both studies reported responses in heavily pretreated patients.
In vitro studies of oxaliplatin have shown its activity in cisplatin-resistant nonseminomatous GCT cell lines. A phase II study of single-agent oxaliplatin in patients with heavily pretreated refractory GCT reported a response rate of 13%. A French series of eight patients with refractory GCT treated with single-agent oxaliplatin reported a response rate of 37%, although how heavily pretreated these patients were is unclear.
Active combination regimens in refractory GCTs
Patients with cisplatin-refractory GCTs have been heavily pretreated by the time they receive third-line therapy, but they tend to be young with a preserved performance status, and therefore the tolerability and response rates of doublet and triplet combination regimens have been studied in this setting ( Table 2 ). A phase II study by the Eastern Cooperative Oncology Group (ECOG 9897) evaluated the combination of paclitaxel and gemcitabine in 28 patients and showed a 21% response rate, including three complete responses. Complete responses in two of the patients were long-term, with disease-free periods 15+ and 25+ months at study publication.
| Drug Regimen | Author | N (Patients) | Responses | Overall Response Rate (%) |
|---|---|---|---|---|
| Paclitaxel/gemcitabine | Hinton et al, 2002 | 28 | 3 PR, 3 CR | 21 |
| Einhorn et al, 2007 | 32 | 4 PR, 6 CR | 31 | |
| Gemcitabine/oxaliplatin | Kollmannsberger et al, 2004 | 35 | 13 PR, 3 CR | 46 |
| Pectasides et al, 2004 | 28 | 5 PR, 4 CR | 32 | |
| De Giorgi et al, 2006 | 18 | 2 PR, 1 CR | 17 | |
| Oxaliplatin/paclitaxel | Theodore et al, 2008 | 26 | 1 PR, 0 CR | 4 |
| Cisplatin/epirubicin | Bedano et al, 2006 | 30 | 8 PR, 9 CR | 57 |
| Oxaliplatin/cisplatin or nedaplatin | Miki et al, 2002 | 18 | 7 PR, 2 CR | 50 |
| Irinotecan/oxaliplatin | Pectasides et al, 2004 | 18 | 3 PR, 4 CR | 40 |
| Irinotecan/paclitaxel/oxaliplatin | Shamash et al, 2007 | 28 | 15 PR, 5 CR | 71 |
| Paclitaxel/cisplatin/gemcitabine | Nicolai et al, 2009 | 22 (5 patients treated in third-line setting) | 8 PR, 0 CR (0 responses in third-line setting) | 36 |
| Gemcitabine/oxaliplatin/paclitaxel | Bokemeyer et al, 2008 | 41 | 19 PR, 2 CR | 51 |
| Necchi et al, 2008 | 19 | 5 PR, 2 CR | 36 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





