Trial
Patients (% GBM)
Treatment
Med. survival (months)
P value
BTSG 66-01 [4]
96 (85 %)
No radiotherapy
3.5
<0.05
WBRT < 5000 cGy
7.7
WBRT ≥ 5000 cGy
8.4
BTSG 69-01 [3]
222 (90 %)
No radiotherapy (BSC)
3.1
0.001
WBRT 5000-6000 cGy
8.4
WBRT + BCNU
8.0
BCNU (no radiotherapy)
4.3
358 (84 %)
CCNU (no radiotherapy)
7.2
NR
WBRT 6000 cGY
8.4
WBRT + BCNU
11.9
WBRT + methyl-CCNU
7.2
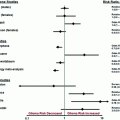
The results of the BTSG 66–01 study led to a subsequent study in which RT was a randomized form of treatment. BTSG 69–01 randomized patients after surgical resection to receive the best supportive care (BSC) or chemotherapy (carmustine/BCNU) with or without WBRT. All therapeutic modalities demonstrated superiorly when compared to BSC with overall survival as a primary outcome. The BTSG investigators also noted that a significant cohort of patients treated with WBRT plus BCNU survived to 18 months, compared with the group receiving RT alone (P = 0.01) [3].
A follow-up study on BTSG 69–01 attempted to further evaluate the role of nitrosoureas plus RT (BTSG 72–01) [6, 14]. Patients received postoperative WBRT with or without a nitrosourea (BCNU or methyl-CCNU); those patients who received BCNU plus WBRT had the longest median survival. BTSG 72–01 prospectively confirmed the survival advantage observed in BTSG 69–01. Additionally, both studies (BTSG 69–01 and 72–01) showed a trend toward improved survival. Again, there was a significant portion of patients who lived up to 18 months in a group that received chemotherapy (BCNU) plus RT. Although the benefit of postoperative RT was clearly established by these two studies, the benefit of adjuvant chemotherapy remained a question.
3 Determining Optimal RT Dose and Fractionation
Optimal radiation doses. A subsequent publication by BTCG retrospectively evaluated combined results from BTSG 66–01, 69–01, and 72–01, with special focus on whether dose escalation of RT improved survival [15]. Altogether, 621 evaluable patients were identified, of which 86 % were pathologically confirmed GBM cases, and the survival data was analyzed by subgroups based on the dose of WBRT received. Median survival times of only 4.2 and 3.1 months were reported for patients treated with less than 4,500 cGy or those who received no RT, respectively. Median survival durations of 6.5, 8.4, and 9.8 months were reported for patients treated with 5,000, 5,500, and 6,000 cGy, respectively. There was progressive improvement in survival with doses in excess of 5,000 cGy, with no statistically significant differences in toxicity observed between the 5,000 and 6,000 cGy treatment groups [15].
This apparent association between improved survival and RT doses ≥5,000 cGy shifted the clinical trial focus to further dose escalation of RT. Salazar and colleagues evaluated doses ranging from 6,000 to 8,000 cGy in three dose levels of WBRT, with and without local boost [16]. More than half the patients randomized to the highest dose level received a cumulative RT dose of 7,500 cGy or more. The study also included a retrospective cohort with then-conventional doses of WBRT (5,000–5,500 cGy). The actuarial median survival in the highest dose cohort (≥7,500 cGy) was 13 months compared to 9.8 months in the next highest dose group and only 7 months in the retrospective cohort treated with then-conventional WBRT. The survival difference between the highest dose cohort and then-conventional WBRT group reached statistical significance (p < 0.05). This statistical result should be interpreted with caution since prospective and retrospective patient outcomes were compared. Survival outcomes between prospectively treated patients at progressively escalated RT doses were not statistically significant, and survival curves for all dose groups were superimposable by 2 years [16].
Within this same study, autopsy data were reported for about 40 % of participating patients, including 10 autopsies from the highest dose cohort [16]. Autopsy specimens demonstrate regions of viable tumor within irradiated regions, even at the highest RT doses of 7,000–8,000 cGy. Additionally, marked radiation effect (e.g., necrosis) was seen microscopically in normal brain tissues at the periphery of the tumor. (Note: The authors did not comment on necrosis in other regions of the brain after WBRT.) These autopsy results, particularly the strong evidence of radiation necrosis at doses exceeding 6,000 cGy, suggested that dose escalation beyond this dose should be undertaken with caution. It is also worth noting that all of these studies were done in the pre-computer tomography (CT) and magnetic resonance imaging (MRI) era and toxicity assessments were largely based on clinical symptomatology and post-treatment autopsy results if available.
In an effort to further define the optimal dosing for post-resection RT (with or without chemotherapy), Chang and colleagues reported results from an intergroup trial evaluating standard WBRT to 6,000 cGy compared with escalated doses of RT [17, 18]. This phase III trial included four treatment arms: (1) WBRT (6,000 cGy), (2) WBRT + boost (6,000 cGy + 1,000 cGy), (3) WBRT (6,000 cGY) + BCNU, and (4) WBRT (6,000 cGy) + methyl-CCNU and dacarbazine (Note: Temozolamide is a prodrug and an imidazotetrazine derivative of the alkylating agent dacarbazine). Unlike the study conducted by Salazar and colleagues that included a retrospective WBRT cohort, the intergroup trial prospectively randomized patients to received then-standard doses of WBRT. In summary, the intergroup trial essentially demonstrated that escalation of RT doses above 6,000 cGy, or the addition of chemotherapy, did not significantly improve survival outcomes beyond WBRT alone to 6,000 cGy (Table 2), and subset analysis of patients with pathologically proved GBM revealed nonsignificant survival differences between the treatment groups (p = 0.59) [18]. Consistent with what has been previously reported in BTSG 69–01 and 72–01, the addition of current BCNU did not significantly improve overall or median survival, with the exception of a trend toward improved survival among the subgroup of patients less than 60 years of age, and a trend toward improved survival at 18 months. In this study, the 18-month survival rate among patients 40–60-years old was 10.3 % for 6,000 cGy WBRT versus 30.9 % for 6,000 cGy WBRT plus BCNU [18].
Med. survival (months) | |||
|---|---|---|---|
Treatment | Patients | Overall | GBM subgroup |
WBRT 6,000 cGy | 141 | 9.3 | 8.7 |
WBRT 6,000 cGy + boost 1000 cGy | 103 | 8.2 | 7.7 |
WBRT 6,000 cGy + BCNU | 156 | 9.7 | 7.8 |
WBRT 6,000 cGy + methyl-CCNU + dacarbazine | 138 | 10.1 | 9.2 |
4 Determining the Optimal RT Field Size
Whole–brain versus involved–field RT. In the early treatments and clinical trials of glioblastoma, WBRT was utilized for treatment primarily because of concerns that glioblastoma may be a multicentric disease in a significant number of cases and that available radiologic techniques were inadequate in determining the extent and location of disease [19–22]. This assumption was subsequently challenged and it was shown that multicentric involvement with GBM is relatively uncommon. For example, Hochberg and Pruitt reported results of serial CT scans and correlative autopsy data in 35 GBM patients [22]. In their report, GBM was found to relapse within a 2 cm margin of the primary site in 90 % of cases, and only 6 % of patients treated with radiotherapy were found to have a multicentric disease at autopsy. Additionally, multiple subsequent studies have demonstrated that there is an upper limit to the WBRT dose in terms of both necrosis and cognitive dysfunction thresholds [23, 24]. Given this toxicity data and its association with high/escalated doses of WBRT is the observed local failure intensification of RT to a local tumor and a surrounding margin.
Beginning in the early 1970s, interest was generated in comparing outcomes of WBRT with involved-field RT (IFRT), where IFRT was defined as radiotherapy administered to the tumor and surrounding tissue encompassed by a 3 cm geometric margin around the tumor [25–28]. In a retrospective review of 127 patients who received RT for treatment of GBM, Onoyama and colleagues reported nearly identical 1-year survival rates with IFRT compared with WBRT [28]. Ramsey and Brand compared two prospectively randomized groups of GBM patients treated with WBRT (median dose = 4,400 cGy) or IFRT (median dose = 5,300 cGy), noting improved survival outcomes in patients treated with higher doses delivered to limited field [27]. In BTCG 80–01, patients with GBM were randomized to receive WBRT to a dose of 6,020 cGy or WBRT to 4300 cGy followed by IFRT boost to additional 1,720 cGy [24]. Survival differences between the treatment groups were not significantly different. Based on these data suggesting comparable outcomes with WBRT and IFRT, IFRT has become the standard of care in the treatment of GBM. This standard persists to this day.
Role of imaging in RT field design. Delivery of RT in the treatment of GBM cases is largely limited by difficulties in target definition/delineation. Although CT and MR imaging have improved the ability to deliver IFRT, these imaging modalities cannot reliably indicate regions of active, non-enhancing, or microscopic tumor. Furthermore, the conventional method used to identify tumor—assessments of gadolinium enhancement on MRI—is also a poor indicator of tumor (or recurrence in the posttreatment setting) after anti-VEGF (vascular endothelial growth factor) therapy, which is increasingly being used to treat patients with glioma. Several promising and novel imaging techniques are being investigated to provide better tumor definition. These will be briefly reviewed below even though their application in treatment planning and posttreatment evaluation varies considerably.
Magnetic resonance spectroscopy imaging (MRSI) is one such technique. MRSI provides information about tumor activity based on the levels of cellular metabolites such as choline, creatine, N-acetylaspartate, lactate, and lipid [29]. MRSI relies on the detection of alterations in these metabolite levels in predicting areas of occult disease; theoretically, targeting of these areas of an occult disease may decrease the rates of local recurrence [30, 31]. In one such early study, Graves and colleagues performed a retrospective study in which the prognostic value of MRSI was explored in patients with high-grade glioma treated with Gamma Knife radiation [31]. Patients without MRSI activity outside the areas of MRI contrast enhancement had significantly better outcomes than patients with MRSI activity outside the region of MRI contrast enhancement. In a follow-up study of 34 patients with high-grade gliomas, Pirzkall and colleagues found metabolically active tumors outside the region of enhancement (≤28 mm) on T2-weighted MRIs in 88 % of patients. Interestingly, MRIs in general predicted a larger volume of microscopic disease by 50 % or more compared with MRSI (using abnormality index of 2, 3, and 4), suggesting that targeted RT based on results of anatomic versus metabolic imaging would likely be of significantly different volumes and locations [30].
Another imaging modality under active investigation is diffusion-weighted MR imaging (DWI). In DWI, each voxel of the image has an intensity that reflects the rate of Brownian motion of water molecules or their diffusion rate in tissue at that location. The intensity of each voxel is quantified by calculating the apparent diffusion coefficient (ADC); that is, the right of water movement in mm2/s. Different tissue types have different ADCs, and increased cellularity correlates with reduced ADC values. Areas of glioma/tumor are hypothesized to have lower ADC values than areas of normal brain, or radiation-induced treatment effects in the post-RT setting. The median ADC values for grade 3 and grade 4 gliomas are approximately 1.5 times that of normal appearing white matter within T2 lesion, with a trend toward lower values within contrast-enhancing lesions [32]. An analysis of the prognosis for 56 patients with untreated glioblastoma showed that both the presurgical values of the 10th percentile of ADC in contrast-enhancing lesions and the volume of the overall T2 lesions that exhibited ADC values less than 1.5 times that of normal appearing white matter were predictive of shorter overall survival [32]. These results are consistent with other published data and with the notion that the presence of regions with ADC values in the range of 1.0–1.5 times that of normal appearing white matter in contrast-enhancing lesions of glioblastoma are associated with a more cellular and aggressive phenotype [33–36]. Immediately after surgery, there are often regions of very low ADC close to the cavity that subsequently become enhancing and then disappear on follow-up examinations. In a recent analysis of 32 patients with GBM who had presurgical, immediate postsurgical, and pre-RT MR examinations, it was found that 21 of 32 patients showed reduced diffusion and 8 subsequently exhibited increased enhancement within a similar region that could have been confused with tumor progression [36]. This implies that the inclusion of diffusion-weighted imaging in the immediate postsurgical scan may be helpful in distinguishing between real and pseudo-progression, and may also be helpful in RT planning. It is also interesting to note that, when the pre-RT examination was taken as a new baseline scan for an expanded cohort patients with GBM, both the volume of the T2 lesion and the volume within the T2 lesion that showed ADC less than 1.5 times that of normal appearing white matter are predictors of poor overall survival, but the volume of the contrast enhancing lesion was not.
Diffusion tensor imaging (DTI) is a more complex version of DWI that can determine the directionality and magnitude of water diffusion, which is termed fractional anisotropy. Values for this parameter lie in the range of 0–1, and are high in normal white matter. DTI quantitates disorganization (damage) of white matter tracts, which is more likely in the lesions or radiation necrosis (or tumor necrosis) than in tumor recurrence because necrosis generally destroys these tracts, while tumor tends to displace or compress them. A case report of three patients found fractional anisotropy values of 0.27–0.29 for recurrent tumor and 0.17 for radiation necrosis, which suggested DTI might be able to distinguish recurrent tumor from necrosis [37]. A larger series will be needed to determine the utility of DTI in diagnosis of pre- and post-radiation enhancing lesions in patients with glioma. Currently, the utility of DTI in radiation treatment planning is unclear.
A number of MRI techniques have been applied to assess changes in microvasculature and to link variations in the estimated parameters with response to therapy. Their role in RT planning is less clear. The two methods most commonly used in the brain are dynamic contrast-enhanced (DCE) and dynamic susceptibility-weighted contrast (DSC) imaging. Several recent reviews have provided a thorough description of the methodology and examples patient data [29]. Briefly, DCE imaging takes advantage of the changes in the T1 associated with the passage of gadolinium through the vasculature and leakage into the extracellular space for regions in which the blood–brain barrier has been compromised [38–42]. When applying certain sampling techniques in conjunction with the latest parallel reconstructions strategies, time resolution of 5–10 s can be achieved for three-dimensional imaging sequence that covers an axial slab of 6–8 cm, partial brain volume. A number of different approaches have been applied to analyze the changes in signal intensity from these dynamic data and to estimate parameters such as the fractional blood volume (f_BV) and permeability (K_ps or K_trans_). The most widely used model is from Tofts and Kermode but other models are also in use today [38].
DSC imaging uses echoplanar sequences with a rapid bolus of gadolinium to assess changes in relaxivity within the vasculature and interstitial space with a 1–2 s time resolution [43]. The change in relaxivity is estimated as being proportional to the concentration of gadolinium. Within a particular region of interest, a decrease in the observed signal intensity usually corresponds to the arrival of the agent in the local vasculature. The changes in intensity are typically characterized by the peak height (PH), area under the curve relative to normal-appearing white matter (rCBV), and the percentage recovery (%REC) or recirculation factor (RF) [44].
Parametric maps that are derived from DCE and DSC imaging data have been proposed as noninvasive methods for predicting a tumor grade and assessing the response to therapy [45–48]. Although the presence of abnormal vasculature is known to be a histologically characteristic marker for glioblastoma, the magnitude and spatial extent of elevated rCBV in the initial presurgery scan were found to be predictive of overall survival [49]. One explanation for this is that, because the surgical resection is focused on the enhancing volume, it typically removes the majority of the region with increased vasculature. For patients with a residual vascular abnormality, conventional treatment with RT and temozolomide exhibits a short-term effect on the lesion, with a reduction in rCBV of a temporary increase in permeability. The magnitudes of these changes are reflected in the size of the contrast-enhancing lesion, with a lesion on the post-RT scan representing a balance between the two effects. In a recent study that followed a cohort of patients with glioblastoma through their initial treatment, it was found that although there was an association between progression-free survival and rCBV at pre-RT and post-RT examinations, none of the vascular parameters were related to overall survival [50]. Modern data that examine the effect of treatment, metabolic or other tumor parameters may be helpful in understanding the relationship between short-term changes in vasculature and long-term effects on the lesion as a whole. The ability to monitor changes in permeability and vascular density is expected to be critically important for the assessment of the impact of anti-angiogenic agents. In such cases, there is an ongoing debate about the most appropriate time points to detect the effect on MR parameters, and whether DCE or DSC techniques should be used to evaluate such changes.
Functional scanning, which uses PET to detect the breakdown of intravenously injected labeled compounds, has shown potential utility for identifying tumor recurrence. However, 18_F–fluorodeoxyglucose (FDG)–PET has limited sensitivity and specificity in distinguishing tumor from necrosis owing to the baseline high glucose utilization of the normal brain. Use of amino acid tracers derived from tyrosine and methionine overcomes the high background signal seen with a glucose–based PET, and can discriminate between tumor necrosis [51]. Furthermore, amino acid transport is energy–dependent and as such requires viable cells. The values of 75 % sensitivity and 75 % specificity were reported for 11_C-methionine PET in a series of 26 patients [52]. Although these novel imaging techniques are of ongoing interest, they are yet to become a standard diagnostic approach in the evaluation and treatment planning of glioblastoma.
5 Dose Intensification: Brachytherapy, Radiosurgery, and Hyperfractionation
In an effort to improve outcomes and glioblastoma, various strategies were employed to locally intensify RT [53–55]. Such strategies have included less traditional forms of RT (brachytherapy, radiolabeled antibodies, radiosurgery), alternative dosing schedules (accelerated and hyperfractionated RT), and the use of radiosensitizing agents. Most of the dose intensification strategies (with the exception of radiosensitizer trials) will be reviewed below.
Brachytherapy. Interstitial delivery of RT, brachytherapy, directs radiation to well-defined tumor target, or resection bed, thereby sparing normal brain tissue from toxicity of high-dose RT and theoretically enabling local, high-dose treatment. Ample research has evaluated different means of delivering interstitial brachytherapy, leading to a debate as to whether radioisotopes should be implanted temporarily or permanently, and which radioisotopes are the most suitable for treatment of gliomas.
Some of the earliest brachytherapy reports from the 1980s focused on the treatment of locally relapsed glioma in patients who had previously received definitive RT [53, 54, 56–58]. Later, focus shifted to using brachytherapy as a local boost in conjunction with IFRT in cases of newly diagnosed glioblastoma [59–63]. A Northern California Oncology Group study (NCOG 6G–82–2) reported a remarkable median survival of 20.5 months in newly diagnosed GBM patients treated with 125_Iodine (125_I) implants following 6,000 cGy of IFRT [59]. The study was criticized for not including a prospectively randomized comparison group of patients who received IFRT alone, and that patients with smaller, more peripherally located tumors were enrolled (e.g., selection bias). Additionally, 38 of the original 67 patients had been ineligible for the brachytherapy boost treatment after demonstrating no response or poor response to the initial IFRT. Consequently, the NCOG study reported on survival outcomes of the highly selected and most favorable patients enrolled in the study.
In contrast, Laperriere and colleagues in a Canadian study failed to demonstrate a significant survival advantage with 125_I implants following standard IFRT to 5,000 cGy [60]. It is difficult to interpret the outcomes of this study since the dose of IFRT was suboptimal. BTCG 87–01 evaluated survival in newly diagnosed malignant glioma patients (grade III and IV) patients treated with combination of BCNU and either IFRT or brachytherapy [64]. Median survival was not significantly different between the treatment groups, and no survival advantage was observed on subgroup analysis of patients with glioblastoma (Table 3).
Table 3
Brachytherapy in newly diagnosed glioblastoma
Trial | Patients | Treatment | Med. survival (months) |
|---|---|---|---|
NCOG 6G-82-2 [59] | 29a | IFRT 6,000 cGy + 125I implants | 20.5 |
Laperriere et al. [60] | 63 | IFRT 5,000 cGy | 13.2 |
IFRT 5,000 cGy + 125I implants | 13.8 | ||
BTCG 87-01 [64] | 270 | IFRT 6,000 cGy + BCNU | 13.7 |
IFRT 6,000 cGy + 125I implants + BCNU | 15.8 |
In aggregate, the favorable survival results reported in single-arm (often single-institution) studies using brachytherapy as part of initial therapy for glioblastoma were not confirmed by randomized studies comparing brachytherapy with IFRT as part of the initial treatment regiment. It is worth noting that brachytherapy is not without complications and any perceived limited benefit needs to be weighed against the risk of potential complications of an invasive procedure. For example, Laperriere and colleagues reported 15 brachytherapy-related complications (out of 63 total patients) in their series, including neurologic decline requiring high-dose steroid treatment, intracerebral hemorrhage, exacerbation of seizures, infection, and arterial occlusion) [60]. Given the lack of prospective randomized study data to support the use of brachytherapy in the initial treatment of glioblastoma, its role in clinical practice (outside of the clinical trial setting) is primarily limited to the treatment of recurrent disease.
GliaSite. The GliaSite RT system (Cytyc) received FDA approval in 2001 as a novel method of brachytherapy delivery for the treatment of high-grade gliomas. The GliaSite is an expandable balloon catheter that is temporarily filled with radioactive 125-I liquid through a subcutaneous reservoir after being placed into the resection cavity after tumor debulking. The balloon applicator conforms to the shape of the resection cavity and theoretically enables homogeneous dose delivery to the surrounding brain tissue. Since the applicator is placed at the time of surgery, there is no need for an additional surgical procedure to perform brachytherapy and, consequently, infection and perioperative risks are theoretically lower than would be expected for more traditional form of brain brachytherapy.
The New Approaches to Brain Tumor Therapy (NABTT) group conducted a trial of GliaSite in the treatment of recurrent malignant gliomas. Patients in the study received 4,000–6,000 cGy of dose to the resection cavity margin (target volume) via the GliaSite system. The observed median survival of 12.7 months was observed in this recurrent setting. These encouraging early results prompted further investigations of the GliaSite system in the upfront or newly diagnosed setting. Most of these more recent trials report survival outcomes that are comparable to historical data of other multi-modality treatments. There are no prospective, randomized studies of GliaSite in the treatment of malignant glioma [65, 66].
Radio–immunotherapy. This unique form of RT delivery involves the use of radiolabeled antibodies targeting malignant brain tissue. Investigators at Duke University have been studying the efficacy of a 131-I-labeled murine anti-tenascin monoclonal antibody (131-I-m81C6) in the treatment of newly diagnosed and recurrent malignant brain tumors [67–79]. Tenascin is an extracellular matrix glycoprotein expressed ubiquitously in multiple tumor types, including high-grade gliomas, but not in normal brain tissue. The murine monoclonal immunoglobulin G2b (81C6) binds to an epitope within tenascin, resulting in inhibition and delay of cell growth. Administration of radiolabeled antibody (131-I-m81C6) involves direct injection of the antibody into the resection cavity at the time of tumor resection.
A phase II study of newly diagnosed glioma patients treated with 131-I-m81C6 followed by conventional IFRT and chemotherapy reported a median survival of 20 months, with a median survival of 18 months in patients with GBM [71]. A more recent study of 131-I-m81C6 in cases of recurrent malignant brain tumors reported a median survival of 15 months in a subgroup of patients with GBM and gliosarcoma [74]. This phase II experience yielded survival results comparable to or more favorable than what has been reported with other salvage therapies, including temozolamide, stereotactic radiosurgery, interstitial chemotherapy, and brachytherapy. In addition, the rates of radiation necrosis in the phase II trials of 131-I-m81C6 were lower than those observed with other dose intensification methods [67]. However, these survival results and rates of neurotoxicity must be interpreted in the context of the overall good performance status of the patient groups analyzed; most patients (>90 %) had Karnofsky performance status (KSP) scores >80. Variations in neurotoxicity can be explained by marked variance in the radiation doses delivered to the 2-cm surgical cavity resection margin [73]. A phase III study is being planned at Duke to follow-up on these encouraging results using patient-specific dosimetry as well as antibody dosing.
Stereotactic Radiosurgery (SRS). Stereotactic radiosurgery involves the precise delivery of high radiation dose in 1–5 treatments. Both frame-based and frameless stereotactic systems were used in the treatment of malignant glioma, with the earliest application being in 1968. Skepticism over the technology and cost constraints resulted in generally slow acceptance by the mainstream oncology community. Traditionally, radiosurgery was delivered with a Gamma Knife device using multiple non-coplanar isocentric 60-Cobalt sources, but more recently, linear accelerator (linac)-based approaches are also becoming popular due to their greater versatility. With the advances in both hardware and software, radiosurgery became increasingly used to treat brain metastases in the 1980s and, shortly thereafter, has also been applied to recurrent glioma treatments.
SRS involves the use of numerous beamlets of radiation aimed precisely at an immobilized target to deliver high-dose, usually ablative dose, of radiation. Although no single beamlet carries significant energy, a large dose is deposited at the intersection of these beamlets, with a steep dose falloff outside the target. As tumor size increases, this falloff becomes shallower and contact surface with the surrounding tissue greater, and typically radiosurgery becomes prohibitive with tumors in excess of 4–5 cm diameter using a single-session treatment. For larger lesions, most practitioners opt to split the treatment up over 3 or 5 fractions, delivering moderate doses at each session. This approach theoretically preserves the biological effectiveness of the treatment while minimizing normal tissue effects in the surrounding brain that would otherwise be unacceptable with single-session treatment to a large target.
Several early retrospective reports of SRS in the setting of recurrent gliomas suggested a survival advantage with the addition of SRS. The suggestion of SRS use in malignant gliomas was first reported by Larson and colleagues from the University of California, San Francisco in 1990 [80]. Subsequently, Loeffler and colleagues from the Joint Center in Boston reported on a 37-patient series where radiosurgery was part of the initial treatment of malignant glioma [81]. After a median follow-up of 19 months, only 24 % of patients died of recurrent tumor (six, all with GBM), whereas two died of complications related to radiosurgery. All others eventually progressed outside of the radiosurgery field. A retrospective study from the University of Maryland comparing survival data in GBM patients treated with IFRT followed by SRS as a local boost treatment or SRS at the time of progression (salvage treatment) found that median survivals favored the group receiving SRS as a boost (25 vs. 13 months; P = 0.0335) [82]. RT Oncology Group (RTOG) study 93–05 evaluated SRS in a randomized study of 203 patients with GBM who received either conventional IFRT (6,000 cGy) plus BCNU or SRS prior to IFRT plus BCNU [83]. This study did not find any significant differences in median survival (13.5 months for SRS vs. 13.6 months for conventional IFRT), 2-year overall survival, quality-of-life deterioration, or cognitive decline [84]. Therefore, outside of the clinical trial setting, there is no clear indication for the use of SRS in the treatment of newly diagnosed GBM.
Fractionated stereotactic radiotherapy (FSRT). Stereotactic radiotherapy involves precisely targeted delivery of radiation using moderate doses over five or more treatments. RTOG 98–03 investigated escalated doses of FSRT in newly diagnosed GBM patients, with patients receiving IFRT to 4,600 cGy followed by FSRT boost to total doses of 6,600–8,400 cGy [85]. The acute- and late-toxicity date in this study were promising (no difference between grade 3 or 4 toxicities) at escalated dose levels of RT. Similar proportions of patients at each dose level required second resections.
Subsequently, the RTOG reported its phase II experience with administering accelerated RT with weekly stereotactic conformal boosts in 76 patients with newly diagnosed GBM (RTOG 00–23) [86]. During the course of standard RT to 5,000 cGy, patients received four weekly FSRT boosts (500 or 700 cGy per fraction), for a total cumulative dose of 7,000–7,800 cGy. Although reported toxicities were manageable, the median survival of 12.5 months was not improved compared with the RTOG historical database [86, 87]. However, a trend for improved survival was observed in subgroups of patients undergoing gross total resection (median survival of 16.1 vs. 12.0 months; p = 0.19). Additionally, a subgroup of patients classified as having more favorable disease according to a recursive partitioning analysis (RPA) model proposed by Curran and colleagues were noted to have improved median survival (14.7 months for RPA class IV patients vs. 11.3 months for the overall study cohort; p = 0.15) [86, 87].
Hyperfractionated and Accelerated Radiotherapy. Hyperfractionation involves more frequent (more than once daily; so-called conventional fractionation) administration of RT doses in an attempt to attain several theoretical radiobiologic advantages, including reduction in late radiation injury and prevention of tumor repopulation between treatments [88, 89]. Additionally, small and frequent doses of RT may redistribute dividing tumor cell population such that some tumor cells can be “forced” to enter more radiosensitive parts of the cell cycle. Thus, hyperfractioned RT (HFRT) offers the potential advantage of being able to give higher cumulative doses of RT without significant added toxicity [88, 89].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree