Fig. 1
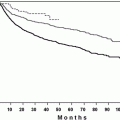
a Kaplan–Meier curve showing progressive improvement in the overall survival of FL patients over time. b Patients diagnosed and treated in post-rituximab era-2 (light color) had a statistically significant improvement in overall survival compared with patients treated in pre-rituximab era-1 (dark color)
Despite improvements in therapy, a number of challenges remain in the management of FL. In addition, it is a disease in which relatively long survival times elevate the importance of quality of life when treatments are considered.
2 Tumor Burden and Clinical Prognostication
The identification of prognostic factors and scoring systems, both clinical and tissue/host based, has helped to refine prognostication for patients with FL and may help guide therapeutic decisions. The Groupe D’Etude des Lymphomes Folliculaires (GELF) and The Follicular Lymphoma International Prognostic Index (FLIPI) scores have emerged as important prognostic models in FL [6, 7]. While the GELF criteria seek to stratify patients by tumor burden and determine when to start cytotoxic therapy, the FLIPI serves as a prognostic model for survival determination. Entry criteria for most clinical trials of untreated FL separate patient groups based on the GELF and/or FLIPI criteria.
The GELF criteria were established in the late 1990s to separate patients into low and high tumor burden based on prognostic factors as noted in Table 1 [7, 8]. In the original 1997 study, patients designated with high tumor burden had a shorter median survival of 71 months compared with the low-risk group whose survival endpoint had not been reached. These criteria have been used to define patient populations who warranted initiation of cytotoxic chemotherapy based on symptoms attributable to their disease/lymphoma or high-risk features (e.g., compression of vital organs) versus asymptomatic patients with low-risk features who were included in “watch-and-wait” (W&W) randomized trials. Over the past two decades, lymphoma groups around the world have used similar, but slightly modified criteria (Table 1) [7–18].
Table 1
Definition(s) of tumor burden in follicular lymphomaa
Modified GELF (PRIMA) [16] | BNLI [10] | GLSG [13] | East German Study Group [17] | ECOG (modified GELF) [18] | |
|---|---|---|---|---|---|
• Any nodal or extranodal tumor mass >7 cm | • Any nodal or extranodal tumor mass >7 cm | • Rapid generalized disease progression in preceding 3 months | • Bulky disease (mediastinal disease >7.5 cm or other mass >5 cm) | • Bulky disease (mediastinal tumor >one-third of thorax or other mass >7.5 cm) | • Nodal or extranodal mass ≥7 cm |
• At least 3 nodal sites, each with a diameter of >3 cm | • At least 3 nodal sites, each with a diameter of >3 cm | • Life-threatening organ involvement | • Presence of B-symptoms | • B symptoms or extranodal manifestation | • At least 3 nodal sites, each with a diameter of >3 cm |
• Any B symptom | • Systemic symptoms | • Renal or macroscopic liver infiltration | • Rapidly progressive disease | • Rapid tumor growth (doubling of the product of the end-to-end diameters measurable lymphoma within 6 months) | • Systemic symptoms or any B symptom |
• Splenic enlargement with inferior margin below the umbilicus line | • Symptomatic splenic enlargement | • Bone lesions | • Cytopenia (hemoglobin level <10.0 g/L, ANC <1.5 × 109/L, and/or platelets <100 × 109/L) | • Cytopenia (granulocyte count <1.0 × 109/L and/or platelets <100 × 109/L) | • Splenomegaly >16 cm by CT scan |
• Compression syndrome (ureteral, orbital, GI) | • Organ compression | • Systemic symptoms or pruritus | • Immunologic phenomena (e.g., hemolytic anemia or immune thrombocytopenia) | • Compression of a vital organ (e.g., ureteral, epidural) | |
• Pleural or peritoneal serous effusion | • Pleural or peritoneal serous effusion | • Cytopenia (Hgb < 10 g/L or WBC < 3.0 × 109/L or platelet counts < 100 × 109/L) | • Leukemic phase (>5.0 × 109/L circulative malignant cells) | ||
• Leukemic phase (>5.0 × 109/L circulative malignant cells) | • ECOG performance status 2–4 | • Cytopenia (Hgb <10.0 g/L, granulocyte <1.5 × 109/L, and/or platelets <100 × 109/L) | |||
• Cytopenia (ANC <1.0 × 109/L and/or platelets <100 × 109/L) | • Serum LDH or β2-microglobulin above normal |
The FLIPI was originally published in 2004, as a prognostic score for newly diagnosed advanced-stage FL [19], then for early-stage disease [20], and also FL in first relapse [21]. An updated report by Federico et al. [6] included the prognostic significance of FLIPI among FL patients treated with rituximab-based treatment (i.e., FLIPI-2). This analysis yielded 3 distinct risk groups with significantly different outcomes (Table 2). The German Low-Grade Lymphoma Study Group (GLSG) also validated the FLIPI risk groups for newly diagnosed high tumor burden FL patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) [11]. An important distinction of the FLIPI is that it is a prognostic scoring system for survival; however, unlike GELF criteria, it is not necessarily a tool to dictate when treatment should or should not be initiated for FL. There is no published data showing that treatment initiation based on FLIPI is associated with enhanced outcomes, especially toward potential improvement in the natural history of the disease. Nonetheless, an adverse FLIPI score (i.e., 3–5) has been integrated into several ongoing clinical trials as selection criteria for initiation of therapy in patients with untreated FL.
Risk group | No. factors | Distribution of patients (%) | 3-year PFS (%) | 5-year PFS (%) |
|---|---|---|---|---|
Low | 0 | 20 | 91 | 79 |
Intermediate | 1–2 | 53 | 69 | 51 |
High | 3–5 | 27 | 51 | 19 |
3 Treatment Approaches
Traditional treatment options for FL include expectant observation for asymptomatic and low tumor burden disease and multiagent cytotoxic chemotherapy for patients who are symptomatic and/or have high tumor burden. Biologic therapy has become an integral part of therapy with agents that target B lymphocytes, such as monoclonal anti-CD20 antibodies and radiolabeled anti-CD20 antibodies. Treatment response to cytotoxic and biologic therapies is high initially; however, with subsequent treatments, response rate and remission duration typically decline and cumulative toxicities increase. The identification of novel targeted agents and new combinations of therapeutics provide the opportunity to continue to improve outcomes for patients with FL.
3.1 Early-Stage Disease
The majority of FL patients present with advanced-stage disease, however, approximately 10–15 % will have stage I disease at initial presentation [22]. For early-stage disease outside of the abdomen and pelvis, definitive radiotherapy (RT) continues to be the recommended approach because of the potential for long-term disease-free survival and possible cure. MacManus and Hoppe [23] evaluated 177 patients with stage I and II NHL who received radiotherapy over median follow-up of 7.7 years. Compared with actuarial survival rates, relapse rates were lower in patients who received RT; 55, 44, 40, and 37 % were relapse free at 5, 10, 15, and 20 years. In addition, results from the Princess Margaret Hospital’s series of involved-field RT (IFRT) for early-stage disease show cumulative relapse rates of 54 and 56 % at 15 and 25 years, with only a 2 % risk of relapse beyond 15 years [24]. The recommended dose is approximately 30 Gray (Gy) for non-bulky disease showing prompt regression and 36 Gy for bulky or slowly regressive disease, in 1.75–2.0 Gy daily fractions. Combined modality therapy has also resulted in excellent disease control [25], and a randomized trial comparing involved-field RT with combined modality therapy is ongoing.
Despite the excellent outcomes associated with RT, the US Lymphocare Study revealed that the majority of early-stage FL patients are either observed or treated with rituximab alone or in combination with chemotherapy, foregoing the potential for cure, even in young patients [22]. In a prior study from Stanford, they had identified 43 patients with early-stage FL who had been observed without therapy [26]. With median follow-up of 7 years, the estimated 10-year survival rate was 85 %, while 63 % of patients had still not required treatment. Thus, there may be select patients with early-stage FL where observation without therapy may be a rationale approach, especially for patients where toxicity concerns of RT outweigh potential benefit.
3.2 Advanced-Stage: Low Tumor Burden
3.2.1 Watch and Wait
A long-standing management of asymptomatic patients with low tumor burden FL has been a W&W approach. Treatment is delayed until the development of symptoms due to lymphoma, the presence of significant cytopenias, and/or if there is compromise of vital organs. In the pre-rituximab era, multiple phase III randomized trials compared immediate chemotherapy with observation for asymptomatic patients with advanced-stage FL showed no difference in OS. In one study, the actuarial chance of patients ages >70 years not needing chemotherapy was 40 % at 10 years [10]. A recently reported European-led registry study studied outcomes of a cohort of 107 low tumor burden FL patients who were managed with a W&W strategy [27]. After an approximate 5-year median follow-up, 50 % of patients had not started on treatment. On multivariate analysis, only involvement of >4 nodal sites correlated with time to lymphoma therapy, while the FLIPI and FLIPI-2 did not. Additionally, the investigators compared freedom from treatment failure (FFTF) and OS among the W&W cohort versus 242 low tumor burden FL patients who received rituximab-containing therapy (initiation of first treatment was not considered an event in the W&W group in order to compare a similar clinical end point), there were no differences in FFTF or OS.
3.2.2 Single-Agent Rituximab
In patients where W&W might hinder quality of life, single-agent rituximab may be considered. In previously untreated FL patients with low tumor burden, the overall response rate (ORR) with rituximab is 47–74 % [14–16], with a median PFS of approximately 2 years without maintenance therapy [14, 16] or 3 years with abbreviated post-induction rituximab [15]. A phase III trial of patients with untreated low tumor burden FL randomized patients to W&W versus immediate induction rituximab therapy (with or without maintenance therapy) was recently reported [9]. At 3 years, 46 % versus 88 % (p < 0.0001) of patients in the W&W group versus maintenance rituximab group did not need treatment. There were no differences in OS noted, although follow-up may be premature to rule out a difference. Interestingly, 78 % of patients in the rituximab induction group did not need treatment at 3 years, which was the same as the maintenance rituximab group.
This latter finding was substantiated in the E4402 Rituximab Extended Schedule or Retreatment (RESORT) study [18]. In RESORT, all 384 low tumor burden FL patients were treated with 4 weeks of induction rituximab and then subsequently randomized to observation or indefinite maintenance rituximab. In terms of the primary end point of time to treatment failure (TTF), rituximab re-treatment was as effective as continual rituximab maintenance. There were no differences in quality of life or OS, while the two arms differed in time to first cytotoxic chemotherapy at 3 years (95 % for maintenance rituximab vs. 86 %, P = 0.03). Altogether, these results suggest that if low tumor burden FL patients are treated with rituximab, 4 weeks of induction therapy are indicated with re-treatment at time of disease progression.
3.3 Advanced-Stage: High Tumor Burden
Several treatment options are available, including single- or multiple-agent chemotherapy with concurrent CD20 antibody therapy; CD20 antibody therapy alone in selected cases; radioimmunoconjugates; and therapy with new targeted therapeutic agents, such as bortezomib (Velcade, Millennium), lenalidomide (Revlimid, Celgene), and idelalisib (Zydelig, Gilead). Autologous and allogeneic hematopoietic stem cell transplantations (SCTs) traditionally are reserved for patients with recurrent or refractory disease, but they may be used earlier in the disease course for patients with a poorer prognosis.
3.3.1 Induction Therapy
The most common treatment for untreated, advanced FL with high tumor burden includes immunotherapy-based treatment with concurrent rituximab and chemotherapy [28]. Several randomized phase III trials evaluating various chemotherapy combinations plus rituximab versus chemotherapy alone have been reported and updated (Table 3) [11, 13, 15, 17, 29–32]. The response rates and either median TTF or median event-free survival (EFS) were superior in all chemoimmunotherapy arms (vs. chemotherapy alone) for chemotherapy-naïve patients and for those who were previously treated. Moreover, OS improvements for the chemoimmunotherapy arms have also been documented.
Table 3
Selected randomized studies of chemoimmunotherapy for patients with FL
Series/author | Year | No. patients | Arms | Conclusions |
|---|---|---|---|---|
Hochster [31] | 2005 | 401 | CVP versus CVP with rituximab post-induction maintenance | Improved PFS (median with rituximab 5.6 years versus 1.8 without maintenance, P < 0.0001) and OS (88 % vs. 72 %, p = 0.03) with post-induction rituximab |
2005 and 2006 | 428 | 8 × CHOP versus R-CHOP (all patients then randomized to IFN maintenance versus autoSCT) | 4-year PFS: CHOP 28 % versus R-CHOP 62 % (p < 0.0001) 4-year OS: CHOP 81 % versus R-CHOP 90 % versus (p = 0.039) | |
Marcus [32] | 2006 | 321 | 8 × CVP versus R-CVP | Median TTF: CVP 15 months versus R-CVP 34 months; OS improved for R-CVP (p = 0.029) |
2006 | 358 | CHVP × 12 over 18 months versus 6 cycles R-CHVP over 6 months (both arms: interferon-alpha × 18 months) | EFS: CHVP 37 % versus R-CHVP 53 % (p < 0.0001); Median OS: CHVP 79 % versus R-CHVP 84 % (p = NS) | |
Herold [17] | 2007 | 358 | 8 × MCP versus R-MCP (both followed by interferon-alpha) | Median EFS (p = 0.001) and median OS (p = 0.0096) improved with R-MCP |
Forstpointner [29] | 2006 | 125 (relapsed) | 4 × FCM versus R-FCM with second randomization of rituximab maintenance (4 weekly doses at 3 and 9 months) versus observation | Response duration significantly prolonged by R-maintenance after R-FCM |
Van Oers [84] | 2006 | 465 (relapsed) | 6 × CHOP versus R-CHOP induction followed by 2nd randomization to rituximab maintenance (one dose q3 months × 24 months) versus observation | Improved PFS with R-maintenance after CHOP induction (HR, 0.30; P < .001) and R-CHOP induction (HR, 0.54; P 0.004) |
Bendamustine plus rituximab (BR) and R-CHOP are the most common first-line regimens, with BR becoming widely used regimen in North America and Europe. Bendamustine was approved in the USA for lymphoid malignancies in 2008, but was first only available in East Germany until 1990. In 2005, Rummel et al. [33] showed BR to be highly active in indolent and mantle-cell lymphoma with ORR of 90 and 75 %, respectively, and complete remission (CR) of 60 and 50 %, respectively. Rummel et al. [34] then showed that BR can be used as first-line therapy in FL due to increased PFS and lower toxicity in their non-inferiority trial. In total, 549 patients were enrolled comparing BR versus CHOP in newly diagnosed, untreated stage III or IV high tumor burden indolent NHL or mantle-cell lymphoma. Superior median PFS was seen with BR versus R-CHOP (69 months vs. 31 months), while there was no difference in OS at follow-up of 45 months. Lower rates of grade 3 and 4 neutropenia, infection, paresthesia, and stomatitis were seen, and there was no alopecia with BR compared with R-CHOP.
Flinn et al. [35] also compared BR to R-CHOP and R-CVP as first-line treatment in the BRIGHT study. BR was found to be non-inferior to R-CHOP and R-CVP in patients with indolent NHL or mantle-cell lymphoma. Although BR had slightly better response rates than R-CHOP/R-CVP (97 % vs. 91 %, p = 0.01), R-CHOP/R-CVP appeared to be acceptable alternatives with similar PFS ands OS rates. Further, there were several toxicities that occurred at a higher rate with BR (i.e., allergic reactions, nausea/vomiting, and skin reactions). R-CHOP may be preferred over BR or R-CVP in situations where patients have a more aggressive histology including patients with FL grade 3b [35]. In the FOLL05 trial, R-CHOP was shown to have better risk–benefit ratio and superior 3 year TTF and PFS compared to R-CVP in the FOLL05 trial [6]. This suggests that the role of an anthracycline in patients with high-risk disease, such as bone marrow invasion and high β2-microglobulin concentration, may be important. R-CVP though is a well-tolerated induction regimen and may be considered especially in patients with concern of cardiac toxicity with anthracycline therapy.
3.3.2 Maintenance Therapy
The concept of maintenance chemotherapy has become a recurrent theme in hematology, which was initially pioneered in pediatric ALL with the POMP regimen that contributed to durable remissions [36]. As FL is an incurable disease, there have been attempts to improve OS and PFS. Initial studies with interferon, chlorambucil, and multiagent chemotherapy showed mixed results and overall unacceptable toxicity. With the integration of anti-CD20 antibodies into clinical practice, there was a renewed opportunity to explore the potential benefit of maintenance therapy following induction treatment for newly diagnosed FL patients.
In a randomized phase III trial known as the Primary Rituximab and Maintenance (PRIMA) study, Salles et al. [16] evaluated the benefit of 2 years of rituximab maintenance in FL after upfront R-chemotherapy (R-CHOP, R-CVP, and R-FCM). With median follow-up of 36 months, rituximab maintenance improved PFS (75 % vs. 58 %), with higher EFS and a higher percentage of patients in CR or unconfirmed CR at 24 months (72 % vs. 52 %). There were higher rates of severe (grade 3/4) adverse events (24 % vs. 17 %) and infection (39 % vs. 24 %) [16]. There was no significant difference in quality of life or OS. In January 2011, the FDA approved use of rituximab maintenance therapy for patients with previously untreated follicular CD-20 positive B-cell NHL who achieve a response to rituximab in combination with chemotherapy.
It is not clear whether these results can be extrapolated to patients treated with other initial chemotherapy regimens. Despite the lack of data, many physicians may administer maintenance rituximab after BR in the hopes of better outcomes than BR alone [37]. In addition, further follow-up is needed to measure long-term toxicities and determine whether the improvement in PFS translates into an OS benefit. If maintenance rituximab is given in a patient with newly diagnosed FL with response to induction therapy, we recommend rituximab to be given every 2 months for a total of 2 years.
4 Radioimmunotherapy
The anti-CD20 radioimmunoconjugates 131I-tositumomab (Bexxar, GlaxoSmithKline) and 90Y-ibritumomab tiuxetan (Zevalin, Spectrum Pharmaceuticals) deliver ionizing radiation to target tumor cells and their neighbors. They are relatively easy to administer, safe, and effective in relapsed/refractory FL [33, 34], including rituximab-refractory disease [35]. Contraindications to their use include thrombocytopenia (i.e., <100 × 109/L) and/or >20–25 % bone marrow involvement with lymphoma. Radioimmunotherapy (RIT) has also been incorporated into the frontline management of patients with untreated indolent lymphoma as a single agent [38] and also through the paradigm of sequential therapy giving one dose of RIT after multiagent induction chemotherapy. Morschhauser et al. reported results of phase III FIT (first-line indolent trial) examining consolidative RIT versus no consolidation in patients who achieved CR or partial remission (PR) following chemotherapy induction [30]. Patients were randomized to one dose of 90Y-ibritumomab tiuxetan or no consolidation. RIT significantly improved median PFS in all patients (36.5 months vs. 13.3 months with no RIT; P < 0.0001). This gain was observed regardless of the depth of initial response to induction chemotherapy, although the benefit appeared to be greater for patients in PR versus CR. A limitation of the FIT trial was that the majority of patients did not receive rituximab-based induction chemotherapy. The extent of benefit of consolidation RIT after combined rituximab/chemotherapy therapy is not clear.
131I-tositumomab also has been incorporated sequentially following chemotherapy [16, 37]. Further information regarding the role of up-front, sequential RIT in indolent NHL should be available soon from the large US intergroup study led by the Southwest Oncology Group (S0016). In that phase III trial, untreated FL patients were randomly assigned to 6 cycles of R-CHOP versus 6 cycles of CHOP followed by 131I-tositumomab.
4.1 Autologous Stem Cell Transplantation (SCT) for First-Line Treatment/Consolidation
Randomized studies that have examined the role of autologous SCT versus non-transplant strategies in the front line setting for FL have shown an improvement in PFS, but without improvement in OS (Table 4). The American Society of Blood and Marrow Transplantation (ASBMT) society does not recommend autologous SCT in the first-line setting for FL based on review of these trials and given the risk of secondary hematological malignancies associated with autologous SCT [39]. A 2012 Cochrane review came to a similar conclusion regarding a consistent improvement in PFS without improved OS [40].
Table 4
Autologous as a first-line consolidative therapy
Reference | Treatment | Number | PFS/EFS | OS |
|---|---|---|---|---|
Horning et al. [85] | Induction: CVP or other Mobil: G-CSF HDT: Cy + VP − 16 + TBI | 37 | Not given | 92 % |
Prospective, nonrandomized | Non-ASCT: not known | 188 (historical) | 88 % | |
Lenz et al. [86] | Induction: CHOP or MCP Mobil: Dexa-BEAM HDT: Cy + TBI | 114 | 65 % (5 year) | Not given |
Prospective | Non-ASCT: CHOP or MCP +IFN maintenance | 126 | 33 % | |
Lenz et al. [87] | Induction: CHOP, R-CHOP Mob: Dexa-BEAM HDT: CY + TBI | 142 (73 %) | 60 % (5 year) | Not given |
Prospective | Non-SCT: CHOP-like and IFN maintenance | 180 (76 %) | 32 % | |
Sebban et al. [88] | ASCT: Induction: CHOP × 4 Mob: CY, VP – 16 HDT: Cy + VP – 16 + TBI | 172 (95 %) | 40 % (7 year) | 76 % |
Prospective | Non-ASCT: CHVP × 6 and IFN maintenance | 167 | 29 % | 71 % |
Ladetto et al. [89] | ASCT: Induction: APO Purging: Rituximab HDT: Mito/Mel | 68 | 68 % | 81 % (4 year) |
Prospective | Non-ASCT: R-CHOP × 6 | 66 | 31 % | 80 % |
Gyan et al. [90] | ASCT: Induction: VCAP (DHAP for salvage) HDT: Cy + f – TBI | 86 | 64 % (9 year) | 76 % (9 year) |
Prospective | Non-ASCT: CHVP and IFN maintenance | 80 | 39 % | 80 % |
5 Relapsed or Refractory FL
Most patients with FL will develop progressive disease including after an initial durable response to immunochemotherapy. Patients with asymptomatic recurrent FL do not require immediate treatment, but need close monitoring for the development of symptomatic disease. There are a number of treatment options for patients with relapsed or refractory FL (Table 5). A number of factors will impact the treatment choice for relapsed disease. These include duration of previous remission(s), type and amount prior therapy used, current tumor burden (at progression), and patient age and comorbidities. Treatment options include immunotherapy alone, immunotherapy combined with chemotherapy, chemotherapy alone, and radioimmunotherapy. Additionally, there should be consideration for consolidation with stem cell transplantation (autologous vs. allogeneic) in select patients.
Table 5
Therapeutic strategies/options for relapsed or refractory FLa
Re-challenge with original therapy (with disease-free remission of >18–24 months)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|