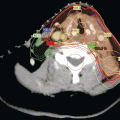
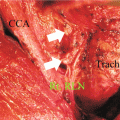
Fig. 2.1
Neck levels and their relation to the accessory nerve
Table 2.1
Incidence and distribution of lymph node metastases by level in patients undergoing a therapeutic lateral neck dissection for papillary thyroid cancer
References | n | Neck level (% involvement) | Comments | ||||
|---|---|---|---|---|---|---|---|
I | II | III | IV | V | |||
Pingpank et al. (2002) [36] | 51 | 12 | 43 | 76 | 59 | 26 | Level I dissected in 31 % |
Kupferman et al. (2004) [35] | 39 | 14 | 52 | 53 | 41 | 21 | Level I dissected in 7 % |
Kupferman et al. (2008) [24] | 70 | 27 | 57 | 62 | 62 | 53 | Level I neck dissection in 43 % |
Yanir and Doweck(2008) [22] | 27 | – | 54 | 68 | 57 | 20 | level I not dissected |
Farrag et al. (2009) [42] | 53 | – | 60 | 66 | 50 | 40 | Level I not dissected |
Yuce et al. (2009) [38] | 46 | – | 46 | 69 | 66 | 34 | Level I not dissected |
Spriano et al. (2009) [39] | 77 | 1 | 38 | 45 | 52 | 8 | Level I neck dissection in 38 %; level V dissected in 69 % |
Lim et al. (2010) [76] | 70 | – | 49 | 74 | 69 | 16 | Level I not dissected |
Nam et al. (2012) [77] | 50 | 0 | 40 | 46 | 42 | 10 | Level I dissected |
Sivanandan and Soo noted involvement of multiple levels in 84 % of their specimens [33]. In other series multiple levels were involved in 60–80 % of patients [35, 36]. Eighty-one per cent of patients presenting after an excisional biopsy were noted to have additional nodes [36]. Discontinual nodal metastases (skip metastases), defined as lateral lymph node metastases in the absence of central lymph node metastases, occurs in 7–19 % of patients [16, 25, 36–38]. With unilateral primary tumours the incidence of contralateral disease in the central and lateral compartments is low, in the order of 0–l5 % [20–22, 27, 39, 40]. Ahmadi and co-workers examined the distribution of nodal metastases in primary and recurrent disease; level IV was more commonly involved in recurrent cases [41].
Recent studies have focused on the involvement of lymph nodes adjacent to the accessory nerve in the anterior triangle (level II) and posterior triangle (level V) [36, 40, 42, 43]. Level II is subdivided by the accessory nerve with the portion above and behind the nerve designated as level IIb (Fig. 2.1) [19]. Pingpank and co-workers noted metastases to level Ilb in 7 (21 %) of 34 neck dissections [36]. In 3 patients level Ilb was the only site of level II involvement. Farrag et al. [42] and Lee et al. [43] described positive nodes in this area in 11.8 and 7 % of dissections, and in both series level IIa was always involved. Level V is subdivided by a plane defined by the inferior border of the cricoid cartilage into level Va superiorly and level Vb inferiorly [19]. Level Va contains the nodes surrounding the accessory nerve. In studies, in which level V has been subdivided, no nodes have been identified in level Va [40, 42].
In summary, based on the surgical management of thyroid cancer, and the surgical and pathological anatomy, two generally accepted anatomical compartments exist—a central compartment and the median visceral compartment, which lies between the trachea and carotid arteries from the hyoid bone to the brachial cephalic vein (level VI). This compartment contains the preglandular nodes, pretracheal nodes, paratracheal nodes, recurrent chain, and the anterior mediastinal nodes. The superior mediastinal nodes are assigned to level VII. The lateral compartment lies between the carotid artery and trapezius muscle and contains the upper middle and lower jugular nodes and the spinal accessory chain of lymph nodes (levels II-V). Regional lymph node metastases from WDTC are frequent and predictable in distribution. The first echelon nodes are the ipsilateral central compartment with subsequent drainage to the lateral neck where metastatic disease is most commonly identified in levels IIb, III, IV and Va. The lymph node metastases are often multiple, and skip metastases are infrequent.
Detection of Cervical Node Metastases and the Role of Diagnostic Imaging
A role for diagnostic imaging in the evaluation of thyroid malignancy has evolved. Examination of the neck is relatively unreliable with reported false-positive and false-negative rates for detection of metastatic disease and nodes of 20–30 % [44, 45]. Imaging studies, including high-resolution ultrasound, CT and MRI are more accurate than physical examination in determining macroscopic node involvement. Current guidelines for the management of WDTC recommend, as the standard, preoperative high-resolution ultrasound of the neck for all patients undergoing thyroid surgery for malignant cytology [19, 46]. The features on ultrasound that are used to determine positive node involvement include size criteria (>13 mm, short access diameter), shape (assessment of the long to short access), and internal architecture (homogeneous and hyperechochic; peripheral punctate calcification; cystic change) [25, 44, 47]. The sensitivity of ultrasound in detecting involved lymph nodes approximates 30 % [48, 49]. Ultrasound is less sensitive to the presence of involved nodes in the central compartment because of the presence of the thyroid gland and the air-filled trachea. [19, 44, 47] Evidence shows that the combined use of ultrasound and CT scan is more accurate than ultrasound alone [47, 50]. Fine-needle aspiration biopsy (FNAB) is appropriate in the evaluation of suspect lymph nodes before surgical management [19]. The iodine load from CT imaging of the neck may alter radioactive iodine uptake in 6 weeks after its administration [44].
Sentinel lymph node biopsy (SLNB) has been evaluated in WDTC [37]. A role for SLNB in WDTC has not been established. In the authors’ opinion the value of this technique would be to identify the subset of patients without occult metastases in which case there would be no controversy with respect to elective management of the neck.
In summary, imaging studies are most useful to assess the lateral compartment. The best way to assess the central compartment of the neck remains surgical exploration.
Impact of Cervical Node Metastases on Prognosis
The high frequency of involvement of cervical lymph nodes in WDTC is well established and regional nodes represent the most frequent site of recurrence [2, 12, 31, 51]. An association of cervical lymph nodes with distant metastases has been observed [9, 12, 21, 52]. It would seem that it should be relatively easy to demonstrate an adverse influence of regional node disease on survival. This has not been the case. The relative, independent and reproducible risk factors for survival, as defined by the EORTC [53] and subsequently the AGES [54], AMES [55] and MACIS [56] prognostic scoring systems, and confirmed in other large retrospective reviews [57, 58], are few. The most consistently important variables influencing survival from differentiated thyroid cancer are advanced age (>45 or 50 years), and the presence of distant metastases. Size and extra-thyroidal extension seem to be reproducible independent variables but with less influence on survival than the first two variables. These variables would not appear to be controversial. Note that regional metastases are not included.
The overall impact of cervical metastases on survival is probably small. Initial reports showed conflicting evidence. It has been observed that the presence of nodal metastases had no effect on either recurrence or survival [3, 55, 58, 59]. Others suggested that lymph node metastases, as they are associated with a higher rate of recurrence, exercise a significant influence on survival [2, 12, 21, 60, 61]. The impact on survival in these series could be attributed to the longer period of follow-up; however, these studies did not adjust the survival analysis by age.
Hughes et al. presented the results of a matched pair analysis comparing 100 patients with lymph node metastases to 100 patients without [62]. They compared ipsilateral N1 nodal disease with those without nodal disease to examine the significance of nodal spread in patients with otherwise equivalent prognostic factors. The 20-year disease-specific survival rates in the N1 and N0 groups were 92 % and 93 %, respectively. Although it did not reach significance, the 20-year survival rate of N1 patients older than 45 years was lower (70 %) than the 20-year survival rates of the age-matched N0 patients (90 %). The overall recurrence rate did not reach significance—17 % and 11 % in N1 and N0 patients, respectively. Age had a major influence in the incidence of disease recurrence, with an 8 % incidence of recurrence in the younger patients, compared with 31 % in the older patients. The authors concluded that the presence of nodal disease was not a significant prognostic factor in patients overall, but its presence did influence a risk of tumour recurrence and mortality in the older patient [62]. Lundgren et al. reported a nested case–control study of a cohort of 5,123 patients with differentiated thyroid cancer treated in Sweden from 1958 to 1987 [63]. The patients were matched by age, gender and calendar period. The mean follow up was 6.7 years. Patients with lymph node metastases experienced a higher mortality (HR 2.5; 95 % CI 1.6–4.1).
A subset of patients with WDTC and node metastases seem to do worse. Older patients are one group, as initially noted by Cady et al. [3] Shaha et al., using a multivariate model, showed that positive nodes significantly influenced survival in patients older than 45 years [64]. Others noted that the prognosis for survival was worse in older node-positive patients [21, 31, 61, 65]. Decreased survival has been associated with mediastinal nodes [2, 31], bilateral nodes [2], a node size of >3 cm [66–68], and extracapsular extension [67]. Simpson and co-workers, in a Canadian survey of 1,074 PTCs and 504 FTCs, found that nodal involvement influenced prognosis in FTC, but not PTC [69].
Leaving aside overall survival, there seems to be a more consistent agreement that the presence of positive lymph nodes does influence recurrence. Leboulleux et al. identified palpable lymph nodes as a significant risk factor for disease recurrence [70]. Other factors associated with recurrence in this series included the number of lymph nodes (>10), extracellular extension, and positive thyroglobulin at 6 months with T4 withdrawal. Wada et al., using a multivariate model in 134 patients with 42 therapeutic and 92 elective neck dissections, established that lymphadenopathy at the time of presentation was significantly related to recurrence [23]. Again, it was observed that local recurrence was impacted by the number of positive lymph nodes. Beasley et al. in a series of 347 patients with stages I and II disease noted that neck node metastases impacted disease-free survival on multivariate analysis [71]. Patients with lateral lymph nodes, multiple level involvement, and superior mediastinal involvement had a worse outcome than those with central compartment involvement alone. The influence of positive nodes at the time of presentation on recurrence has been emphasized in other series [2, 12, 21, 27, 62, 65]. Randolph and co-workers recently challenged the paradigm of assigning the same magnitude of risk to all patients with N1 disease [72]. In their study, the recurrence rate for patients staged N0 at presentation was 2 % compared with 22 % for those who are initially N-positive. They noted that in pN1 patients the recurrence rate was impacted by the number of positive nodes—4 % with <5 nodes and 19 % with >5 nodes. Extranodal extension was associated with a median risk of recurrence of 24 % [72]. A scoring system to stratify patients by risk of lymph node recurrence has been proposed by Ito and co-workers [73]. One point each was assigned to the following: (i) age of >55 years; (ii) male gender; (iii) massive extrathyroidal extension; and (iv) tumour of >3 cm diameter. The 10-year regional disease-free survival was 98.4 % with a score of 0. Survival decreased incrementally to 64.7 % with a score of 4. The use of molecular markers may help predict recurrence in the future [74].
In summary, the predominant opinion today is that local node metastases increases the risk of local recurrence and cancer-specific mortality in older patients (>45 years), especially if the nodes are bilateral, involvement of mediastinal nodes, and if the nodes are fixed, or if there is tumour invasion through the capsule of the lymph node [46]. This notion is reflected in the staging system for thyroid cancer, in which for patients 45 years or older, the presence of lymph node metastases (N1) upgrades stage I or II to stage III [75]. Despite the frequency of microscopic lymph node involvement (60–90 %) only 5–15 % of patients with PTC in whom no prophylactic neck dissection has been performed develop clinically significant lymph node metastases at a later date [25].
An Overview of Management
Careful and appropriate surgery is the most important component in the treatment of WDTC. The extent of surgical resection depends on the extent of cancer with the aim to control the cancer and avoid re-operation, if possible with minimal morbidity. Badly treated thyroid cancer can be a progressive and recurrent disease, whereas with expert management it is readily controlled. Local recurrence and complications of surgery are equally important in determining the timing and extent of treatment of the neck. For this reason the management of the central and lateral compartments of the neck is considered separately. Current guidelines for the management of the neck in patients with WDTC are shown in Table 2.2.
Table 2.2
Guidelines for the treatment of the neck in WDTC
Central compartment (level VI) Recommendation 27 | Lateral compartment (levels II-V) Recommendation 28 |
|---|---|
(a) Therapeutic central compartment (level VI) neck dissection for patients with clinically involved central or lateral neck lymph nodes should accompany total thyroidectomy to provide clearance of the disease from the central neck. Recommendation rating: B | Therapeutic lateral compartment lymph node dissection should be performed for patients with biopsy-proven metastatic lateral cervical lymphadenopathy. Recommendation rating: B |
(b) Prophylactic central compartment neck dissection (ipsilateral or bilateral) may be performed in patients with papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes, especially for advanced primary tumours (T3 or T4). Recommendation rating: C | |
(c) Near-total or total thyroidectomy without prophylactic central neck dissection might be appropriate for small (T1 or T2), noninvasive, clinically node-negative PTCs and most follicular cancers. Recommendation rating: C |