Fig. 3.1
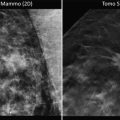
Lobar growth pattern in diffusely growing in situ breast carcinoma. (a) Magnetic resonance imaging, (b) the same image with diseased area marked, (c) corresponding large-format histology section of the lesion with diseased area marked. Note the lobe-like shape of the lesion and involvement of a single lactiferous duct. The radiology images are courtesy of Dr. Mats Ingvarsson. c was reproduced from ref. [52]
The development of in situ carcinomas may be a synchronous or asynchronous process. If synchronous, the entire sick lobe, a segment of the sick lobe, or many terminal units show histologically detectable in situ carcinomas at an early phase of cancer development. In such cases, the area/tissue volume involved with malignant cells (i.e., the extent of the disease) is similar during the early and later phases of the cancer’s natural history (though the involved lobe, segment, or terminal units may grow during the process). If the malignant transformation is asynchronous, only a few structures will show histologically detectable signs of malignant transformation at the beginning of the process, but these signs will be more widespread at the end of it. In this case, the extent of the disease also increases with time.
Early Invasive Cancer
Further mutations in the malignant cells and cells of the surrounding tissue may lead to deregulation of the epithelial–stromal balance, resulting in the cancer cells losing their ability to rebuild the ductal-lobular architecture and becoming unable to reproduce the myoepithelial layer and basal membrane. Individual cancer cells and their groups will come into direct contact with the stroma, which has also undergone remodelling during this process. The remodelled stroma limits the lesion to an altered part of the breast tissue called the invasive tumour focus [35]. Such a focus may develop from the in situ cancer at a single place within the breast (unifocal invasive component) or on several distant loci (multifocal invasive component, primary type). Cancer cells may enter prelymphatic [36] and lymphatic spaces and be transported through these channels to distant parts of the breast tissue, as well as locations outside the breast, such as the lymph nodes. The transported cancer cells may be dormant for years and decades, but they may also leave the channel, interact with the surrounding tissue, and develop secondary invasive foci. If this process is localised to the breast, multifocal invasive component(s) of secondary (metastatic) type develops. Of course, in this case, the process no longer respects the boundaries of a sick lobe.
In most cases, invasion appears focally in the sick lobe, and the vast majority of breast carcinomas exhibit both in situ and invasive components. The real extent and distribution of the lesions in the tumours can only be appreciated if the parameters of both in situ and invasive components are combined [33].
Advanced Cancer
Through the proliferation of the cancer cells, the invasive component(s) of the tumour may grow, and the tumour foci may eventually coalesce, resulting in a larger tumour mass and more complex morphology. With further mutations and dedifferentiation, new cell clones may appear within the cancer, leading to intratumoural and intertumoural heterogeneity in tumour characteristics. The transported cancer cells may wake from their dormancy and develop additional tumour foci within the breast or metastases in other organs. Through these mechanisms, the cancer gradually enters the advanced phase.
On rare occasions, the remodelling of the stroma is insufficient and the growth-limiting effect of such remodelling is missing. In these cases, invasion may occur simultaneously at many places in proximity to the in situ component. The tumour cells infiltrate the normal stromal structures in an unlimited way, giving rise to a spider web-like invasive component, which is usually extensive (diffuse invasive component) [37].
An advanced invasive carcinoma with a diffuse in situ component showing a lobar growth pattern is illustrated in Fig. 3.2.
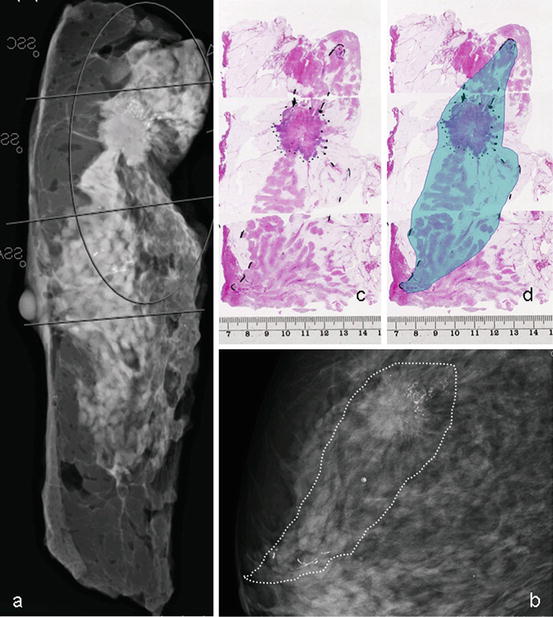

Fig. 3.2
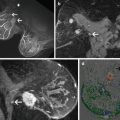
Breast carcinoma with a unifocal invasive component and diffusely growing in situ component occupying a whole breast lobe. (a) Radiogram of a slice of the mastectomy specimen, (b) mammogram of the lesion with diseased area marked, (c) corresponding large-format histology sections (half of the slice reconstructed in three adjacent standard-sized large slides), (d) the same histology sections with diseased area marked. Note the lobe-like shape of the lesions and involvement of a single lactiferous duct. The radiology images are courtesy of Dr. Mats Ingvarsson
The Clinical Relevance of Breast Cancer Subgross Morphology
Distribution of the Cases by Size, Extent, and Focality
Purely in situ carcinomas comprise 10–20 % of breast cancer series in countries with ongoing mammography screening. In our material (Central Hospital Falun, Sweden, period 2008–2011), the proportion of in situ cancer was 14 % (107/780) of the consecutive series of newly diagnosed breast cancers. Half of the cases were extensive (52 %, 55/107), in that they occupied a tissue volume measuring at least 4 cm in the largest dimension, and half of them were non-extensive (48 %, 52/107). The lesions were unifocal in 30 % of cases (32/107), multifocal in 30 % (32/107), and diffuse in 40 % (43/107) (Table 3.1).
Table 3.1
Distribution of cases in a consecutive series of newly diagnosed breast carcinomas in Falun, Sweden, 2008–2011 by tumour size, disease extent, and focality
Unifocal | Multifocal | Diffuse | Total | ||||
|---|---|---|---|---|---|---|---|
Extensive | Non-extensive | Extensive | Non-extensive | Extensive | Non-extensive | ||
In situ | 0 | 30 % (32/107) | 20 % (21/107) | 10 % (11/107) | 32 % (34/107) | 8 % (9/107) | 100 % (107/107) |
Early Invasive | 0 | 41 % (107/260) | 18 % (48/260) | 15 % (38/260) | 21 % (55/260) | 5 % (12/260) | 100 % (260/260) |
Advanced | 3 % (13/413) | 32 % (133/413) | 29 % (118/413) | 8 % (35/413) | 23 % (96/413) | 5 % (18/413) | 100 % (413/413) |
Total | 36 % (285/780) | 35 % (271/780) | 29 % (224/780) | 100 % (780/780) | |||
In addition to purely in situ carcinomas, microinvasive cancers (invasive foci less than 1 mm in size) and invasive carcinomas less than 15 mm size belong to the category of early breast carcinomas, as we define [30, 38]. Patients with such tumours have an excellent, over 90 %, 10-year cumulative survival. The proportion of cases in our material that were classified in this category was 48 %: 14 % (107/780) in situ, 33 % (260/780) invasive <15 mm, and two cases of microinvasive cancer. The majority (70 %, 182/260) of the early invasive cancers had a unifocal invasive component, but when the combined morphology of the in situ and invasive components were taken into account, the majority (59 %) were in fact multifocal or diffuse; 40 % (103/260) of the early cases were extensive and occupied a tissue volume measuring 4 cm or larger (Table 3.1).
More advanced cancers have an invasive component measuring 15 mm or greater. Patients with such tumours have less favourable survival outcomes compared to early breast cancer cases. The proportion of cases in our material that were classified in this category was 52 % (413/780). Approximately one-third (35 %, 126/413) had unifocal combined (in situ + invasive) morphology, one-third (37 %, 153/413) had multifocal, and the rest (28 %, 13/413) had diffuse combined lesion distribution; 55 % (227/413) of the cases were extensive and occupied a tissue volume measuring 4 cm or larger (Table 3.1).
In summary, most breast carcinomas exhibit both in situ and invasive components. Although up to 70 % of invasive tumours have only a unifocal invasive component, most breast carcinomas have complex morphology when the distributions of the in situ and invasive components are combined. This complexity is already evident at early stages of the disease. Half of breast cancer cases are extensive and occupy a tissue volume measuring 4 cm or larger in the greatest dimension. This conclusion is in concordance with the results of whole organ studies [24, 39–42], studies using large-format histology in routine diagnostics [23, 32–34, 43–48], and studies relying on modern radiological breast imaging methods [9, 28, 29].
Local Disease Control
Ipsilateral local recurrences following breast-conserving surgery develop most often in the vicinity of the operative area. Postoperative irradiation decreases the incidence of such recurrences; without irradiation this incidence may be as high as 40 %. A recent meta-analysis of more than 10,000 women treated with breast-conserving surgery found that 35 % of patients without and 19.3 % with postoperative irradiation had local recurrences during the 10-year follow-up period [49]. A long-term (25-year) follow-up study in the USA demonstrated a more than six times higher locoregional recurrence rate in patients treated with breast-conserving surgery with postoperative irradiation compared to those treated with mastectomy [50]. In addition to the possibility of incomplete surgical intervention, the recurrence can be explained by the possibility of developing additional malignant foci in the at-risk tissue in which no malignancy was detected at the time of surgery. This possibility clearly indicates the necessity of removing or destroying the entire at-risk tissue within the breast, the entire sick lobe.
According to our previously published series with long-term follow-up, the rates of local recurrence after breast-conserving surgery were almost ten times higher in extensive compared to non-extensive in situ carcinomas of non-special type (19 % versus 2 %). The highest rates were seen in cases of diffuse in situ carcinoma, especially those with signs of ductal neogenesis (27 % recurrence rate) [5]. Similar results were demonstrated by Silverstein and Lagios [51], who showed that mastectomy is needed for the majority of extensive (40 mm or larger) in situ carcinomas to reach a <20 % 12-year local recurrence rate.
According to our previously published series, extensive breast carcinoma (all size categories included) had an almost triple relative risk of ipsilateral local recurrence after breast-conserving surgery and radiotherapy compared to non-extensive cases (RR, 2.7511; CI, 1.3401–5.6478). Likewise, the relative risk of local recurrence was tripled if breast-conserving surgery was performed compared to the risk in mastectomy cases (RR, 2.8182; CI, 1.1955–6.6435) [52]. These results were confirmed by data on disease-free survival in the same series of patients [53]. Thus, our findings indicate the need for mastectomy in extensive breast cancer cases, comprising 40–50 % of the series, which is in concordance with the results of some previous publications on this topic [54, 55]. The introduction of more sensitive preoperative imaging methods, such as magnetic resonance imaging, which allows more precise mapping of the disease, increases mastectomy rates, from 29 to 52.8 % in one study [28]. As magnetic resonance imaging correlates well with histologically detectable malignant structures, this increase in the mastectomy rate is justified.
Using preoperative ductoscopy to find the altered lactiferous duct and mark its branches efficiently assists the surgeon in delineating the area to excise. Such an approach has substantially reduced the incidence of local recurrences [56]. As a special ultrasound approach, ductal echography is also efficient in delineating the sick breast lobe [9]. The concept and the technical details of lobectomy as a successful alternative surgical approach in breast cancer are presented by Giancarlo Dolfin elsewhere in this book.
Survival
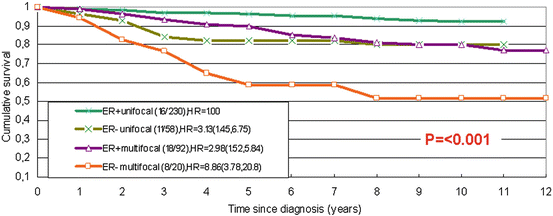
Despite the influence of the in situ component, survival is mainly related to the characteristics of the invasive component of the breast cancer. The worst outcome is related to diffuse invasive breast carcinoma [37, 57], but multifocality of the invasive component and the extent of the disease also impact prognosis. Most related publications have reported a greater propensity of metastatic spread to the axillary lymph nodes in multifocal invasive carcinomas compared to unifocal tumours [33, 47, 48, 58, 59]. Our studies have demonstrated that the risk of lymph node metastasis is approximately doubled in multifocal and tripled in diffuse invasive carcinomas compared to unifocal carcinomas [33, 47, 48].
Relatively few studies have been performed to examine the effect of multifocality on breast cancer-related cumulative survival. These studies generated somewhat conflicting results, as the diagnostic criteria and understanding of the multifocality of the process varied among the studies. Some publications have reported a significant influence of multifocality on disease-free survival in breast cancer patients but no significant effect on overall survival [60]. However, four recent independent studies reported a highly significant impact of breast cancer multifocality, which reduced the overall survival of the patients [57, 61–63]. Such an impact of multifocality seems to be independent of other morphological prognostic parameters [57, 62, 63] and the applied therapeutic measures [62]. The impact of tumour multifocality on the cumulative survival of oestrogen-receptor positive and oestrogen-receptor negative patients is illustrated in Fig. 3.3.