Radiation therapy plays an integral role in the management of melanoma. Radiation therapy is infrequently needed for the treatment of the primary site, but there are occasions when it is appropriate. The role of adjuvant radiation therapy to resected nodal basins is becoming clearer from recent randomized data for patients at high risk. The role of radiotherapy combined with radiosensitizers, biologic or with hyperthermia, continues to evolve. Radiation therapy plays a vital role in the treatment of brain metastases and is useful for other systemic metastases. Emerging technology such as stereotactic radiation therapy may be useful in achieving durable palliation for selected patients.
Surgery, systemic therapy, and radiation therapy all play an important role in the management of cutaneous melanoma. Depending on the clinical situation, radiotherapy may be used to improve local control or for palliation. This review describes the experience with radiation for a variety of clinical indications. Specifically, the roles of radiation as definitive and adjuvant therapy to the primary site, as an adjuvant after lymph node dissection, and to the lymph node basin in the absence of complete node dissection are discussed. The use of concurrent radiation therapy with interferon, chemotherapy, and hyperthermia are also described. The rationale behind radiation dose and fractionation schemes is explained. Radiation therapy as a palliative modality in stage IV melanoma is explored, including treatment of brain, subcutaneous, and other metastases. The rationale behind choosing whole brain radiation, stereotactic radiosurgery for brain metastases, and stereotactic radiation therapy for oligometastatic disease is specifically described.
Definitive and adjuvant radiotherapy to the primary site
Wide resection remains the primary initial treatment of cutaneous melanoma. In rare cases, such as inoperability because of medical comorbidities or other reasons, definitive radiation may be considered to the primary site. There are limited data on the role of radiation therapy alone as primary management for invasive melanoma. In one series of 95 cases of invasive melanoma, patients received high doses of radiation with superficial x-ray therapy. Five-year survival was close to 70%. There is considerably more experience using radiation therapy in the treatment of lentigo maligna or lentigo maligna melanoma. In these series, superficial x-rays have generally been used, with local control rates of greater than 90% reported. However, radiotherapy is generally not used in the treatment of lentigo maligna for several reasons. These include innovations in surgical techniques, the emerging use of topical therapies such as imiquimod, and difficulties in designing radiotherapy fields in cases in which margins are poorly demarcated.
Generally, the rate of local recurrence in invasive cutaneous melanoma is less than 5% after adequately wide excision of the primary site. However, in certain circumstances local recurrence at the primary site is unacceptably high after surgery alone, and adjuvant radiation therapy may be considered to improve local control. Occasionally, anatomic constraints may limit the ability to obtain widely negative margins, particularly in the head and neck. Radiotherapy has been effectively used in the setting of either positive or close margins where further reexcision was not considered feasible. Key risk factors for local recurrence are thick tumors (Breslow>4 mm), ulceration, and the presence of satellitosis and/or angiolymphatic invasion. In our practice, the presence of satellitosis is a particularly high-risk feature for local recurrence, and adjuvant radiation to the primary site is often used. Once local recurrence does occur, the risk for further recurrent disease at the primary site increases greatly. In these patients, we typically recommend reresection followed by postoperative radiation therapy.
Adjuvant radiotherapy to the primary site: desmoplastic melanoma
Desmoplastic melanoma is a rare subtype of melanoma accounting for approximately 1% to 4% of all melanomas. It is characterized by variably pleomorphic, spindle-shaped cells with associated collagen production. Clinically, the lesions often appear to be amelanotic. This subtype is frequently associated with perineural spread, so-called neurotropism, and has been associated with increased local failure rates.
Although some investigators have shown local failure rates in desmoplastic melanoma to be in the 20% to 50% range after surgery alone, a series of 280 patients from Australia found the rate to be on the order of only 10%, with higher recurrence rates noted with the presence of neurotropism and when surgical margins were less than 1 cm. Some have advocated adjuvant radiation therapy to the primary site in all cases of desmoplastic melanoma. In contrast, others have reported local failure rates of less than 5% with surgery alone and advocate close monitoring for disease recurrence.
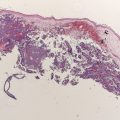
Although prospective data regarding the role of radiotherapy in desmoplastic melanoma are lacking, we currently offer postoperative radiation for tumors that exhibit perineural spread and/or for lesions thicker than 4 mm, based on retrospective reports showing improvement in local control. In addition, radiotherapy should be considered in desmoplastic melanoma that has not been resected with wide margins and in locally recurrent lesions. Fig. 1 shows an electron beam setup for a patient with desmoplastic melanoma. Indications to treat were the location on the scalp and depth of invasion (>4 mm). The patient was placed in a prone position, and the outlined radiation field included the scar with a generous margin. An immobilization mask was constructed and bolus was placed to increase the skin dose. We treated the area with simple en face electron beam technique delivering 60 Gray (Gy; 1 Gy equals 100 rad) to the area in 30 fractions at 2 Gy per fraction.
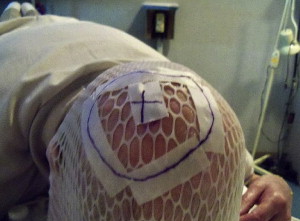
Adjuvant radiotherapy to the primary site: desmoplastic melanoma
Desmoplastic melanoma is a rare subtype of melanoma accounting for approximately 1% to 4% of all melanomas. It is characterized by variably pleomorphic, spindle-shaped cells with associated collagen production. Clinically, the lesions often appear to be amelanotic. This subtype is frequently associated with perineural spread, so-called neurotropism, and has been associated with increased local failure rates.
Although some investigators have shown local failure rates in desmoplastic melanoma to be in the 20% to 50% range after surgery alone, a series of 280 patients from Australia found the rate to be on the order of only 10%, with higher recurrence rates noted with the presence of neurotropism and when surgical margins were less than 1 cm. Some have advocated adjuvant radiation therapy to the primary site in all cases of desmoplastic melanoma. In contrast, others have reported local failure rates of less than 5% with surgery alone and advocate close monitoring for disease recurrence.
Although prospective data regarding the role of radiotherapy in desmoplastic melanoma are lacking, we currently offer postoperative radiation for tumors that exhibit perineural spread and/or for lesions thicker than 4 mm, based on retrospective reports showing improvement in local control. In addition, radiotherapy should be considered in desmoplastic melanoma that has not been resected with wide margins and in locally recurrent lesions. Fig. 1 shows an electron beam setup for a patient with desmoplastic melanoma. Indications to treat were the location on the scalp and depth of invasion (>4 mm). The patient was placed in a prone position, and the outlined radiation field included the scar with a generous margin. An immobilization mask was constructed and bolus was placed to increase the skin dose. We treated the area with simple en face electron beam technique delivering 60 Gray (Gy; 1 Gy equals 100 rad) to the area in 30 fractions at 2 Gy per fraction.
Radiotherapy to the regional lymph node basin
Adjuvant Radiotherapy to the Regional Node Basin After Lymph Node Dissection
Multiple retrospective series have reported apparent improvement in regional control in high-risk resected nodal basins with the use of postoperative radiotherapy. These studies have consistently shown high-risk factors for regional failure to include multiple positive nodes (>3 nodes and especially ≥10 nodes), 1 or more nodes of large size (>3 cm), extracapsular extension of clinically palpable disease, and/or regionally recurrent disease, with combinations of these factors conferring the greatest risk.
The addition of radiotherapy after modified or radical neck dissection has consistently been reported to result in regional control rates upwards of 90% in clinically node-positive patients. The morbidity of treatment is potentially significant and includes up to a 10% rate of complications at 5 years, including hearing loss, wound breakdown, bone exposure, and ear pain. In cases of bilateral nodal metastasis, as seen occasionally arising from midline head and neck and scalp primary sites, bilateral cervical radiation has been associated with higher complication rates; a 50% rate of complications at 12 months was reported in one series.
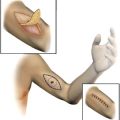
In the axilla, regional recurrence rates of 50% or more have been reported after axillary node dissection in patients with extracapsular extension and/or 2 or more nodes involved. Fig. 2 shows the treatment of the axilla in a patient who had undergone axillary dissection for a large tumor-containing node with extracapsular extension. After adequate recovery from surgery, postoperative radiotherapy was delivered with intensity modulated radiation therapy (IMRT) delivering standard fractions of radiation at 2 Gy per day to a total dose of 60 Gy in 30 treatments. Care was taken to minimize hot spots greater than 60 Gy to protect the brachial plexus, and the lungs and humeral head were contoured as avoidance structures.
In the axilla treated with postoperative radiotherapy, side effects may be seen in up to 30% at 5 years, with the most significant being lymphedema. The inguinal region is the nodal basin at greatest risk for lymphedema and other complications when node dissection and radiotherapy are both used. There is also evidence that patients with body mass indices greater than 30 kg/m 2 are at even higher risk for treatment-related complications (up to 80%) after combined therapy in the groin. Given the higher chance for toxicity in the groin, we are cautious when recommending radiation to this nodal basin. Although the criteria used generally remain the same as in the other basins (ie, offered to those with multiple positive nodes, large nodes, extracapsular extension, or recurrent disease), a more careful risk and benefit assessment is required. Thus, we tend to be more conservative in recommending radiation to the groin, particularly in patients with significant postoperative edema, high body mass index, and those considered to be at a lower risk for recurrence.
Data from an older prospective randomized trial evaluating the role of radiation after the resection of nodal metastasis was inconclusive. This study may be criticized for many reasons, including the use of low-energy x-rays, planned treatment breaks, a low total dose of radiation, and a daily fraction size less than 2 Gy per day. More recently, prospective trial data from Australia have been reported showing improved regional control with adjuvant radiation therapy in patients at high risk of regional failure. In an initial phase II trial, 234 patients received 48 Gy in 20 fractions at a dose of 2.4 Gy per fraction to either the head and neck, axilla and supraclavicular basin, or the ilioinguinal basin. Eligible patients had more than 1 node involved with melanoma, 1 node with extracapsular extension, regional basin recurrence, or reported intraoperative tumor spill. Regional in-field recurrences were noted in only 7% of treated patients, much less than anticipated from historical data, leading to a subsequent prospective randomized trial by the same group. Inclusion criteria for this trial included 1 or more of the following: parotid nodal involvement; at least 2 cervical or axillary or 3 inguinal nodes involved by tumor; 1 node at any site with extracapsular extension; or 1 involved node measuring at least 3 cm in greatest diameter in the head and neck or axilla or 4 cm in the groin. After lymphadenectomy, patients were randomized to receive radiation to the regional nodal field (48 Gy in 20 fractions) or observation (123 patients in the radiation arm and 127 in the observation arm). The rate of regional recurrence as the first relapse was the primary endpoint, with overall survival as a secondary endpoint. Although overall survival was not different for the 2 groups, there was a statistically significant decrease in nodal basin recurrence with the addition of radiotherapy: 20 patients (16.3%) recurred within the treatment field in the radiation arm versus 34 patients (26.8%) in the observation arm ( P = .04, Table 1 ).
| Author | Inclusion Criteria | Treatment Arms | Patients (n) | Median Survival (mo) | Regional Failure | Median Time to Recurrence (mo) |
|---|---|---|---|---|---|---|
| Creagan et al (1978) | Any node-positive melanoma | S+RT S | 27 29 | 33 22 ( P = .09) | 3 patients 1 patient | 20 9 ( P = .07) |
| Burmeister et al (2009) | ECE or node size >3 cm or multiple positive nodes | S+RT S | 123 127 | 31 47 ( P = .14) | 20 patients 34 patients ( P = .041) | Not reported |
In summary, prospective randomized data have now validated criteria that identify patients with a substantial risk of regional recurrence after complete lymphadenectomy and shown a benefit in regional recurrence for the addition of radiotherapy for patients with these high-risk features. It will be important to see more detailed analysis of these study results to help refine the selection criteria for postoperative radiation (ie, to determine whether all of the criteria used were equally predictive of regional relapse), understand the long-term toxicity of therapy, and, perhaps most importantly, evaluate the success of salvage radiation therapy for those patients who failed in the regional basin after surgery alone. If overall survival is the same and ultimate regional control is also similar for patients treated with postrelapse versus routine postoperative radiation, then the risk/benefit ratio would most likely favor reserving nodal basin irradiation until the time of regional relapse. At present, a careful assessment of the risks and benefits of therapy is required to select appropriate patients for postoperative radiation to dissected nodal basins, but this approach can be used with the knowledge that there is strong evidence of improved regional control compared with surgery alone.
Radiotherapy to the Lymph Node Basin in the Absence of Complete Dissection
Some investigators have advocated the delivery of nodal basin radiotherapy instead of complete node dissection, particularly in patients with metastatic melanoma to cervical nodes from head and neck primary sites. A series of 36 patients has been reported in which patients underwent cervical nodal basin irradiation after an excisional biopsy established the presence of nodal involvement. Treatment delivered 30 Gy at 6 Gy per fraction twice per week (5 treatments total). The 5-year rate of complications was reported as less than 10% and the 5-year regional control rate was greater than 90%.
Elective radiation to the clinically node-negative regional basin after wide excision of primary cutaneous melanoma of the head and neck greater than 1.5 mm in thickness or Clark level IV has been evaluated. Outcomes for 157 patients treated with this approach from 1983 to 1988 were reported in 2004. Patients with desmoplastic melanoma and those who had undergone surgery for suspicion of lymph node metastasis were excluded. Ten-year locoregional control was reported as 86%.
Despite these results, the current standard of care in the treatment of melanoma includes wide excision of the primary site and sentinel lymph node biopsy for the appropriately selected patient. When the sentinel nodes are shown to contain malignancy, completion node dissection is recommended. However, circumstances may arise in which sentinel biopsy is not feasible for either medical or technical reasons. In these rare cases, we currently follow the regional basins with clinical examination and serial ultrasonography, but regional nodal irradiation is an alternative that can be considered in selected patients.
Radiotherapy with Concurrent Interferon
Although controversial, there is strong evidence that interferon α-2b improves relapse-free survival and even overall survival in stage III melanoma. In addition, there may be a theoretic synergy and radiosensitization with the combination of radiation and interferon. Several studies have evaluated the role of concurrent radiotherapy and interferon or radiotherapy within 1 month of the delivery of interferon in resected, high-risk, stage III melanoma. These studies have generally reported locoregional control to be comparable with historical rates. However, despite small numbers of patients in these series, most have reported greater than expected late toxicity, particularly soft tissue injury, lung injury, and myelitis.
The use of radiotherapy with concurrent interferon has been prospectively evaluated at our center and results recently reported. Inclusion criteria included stage III nodal disease involving axilla, neck, or groin, with 4 or more positive nodes or a single node of 4 cm or larger, or with microscopic or macroscopic extracapsular extension involving more than 10% of the capsule circumference. Induction interferon α-2b, 20 million U/m 2 /d, was administered intravenously for 5 consecutive days every week for 4 weeks. Subsequently, radiation of 30 Gy in 5 fractions, 2 fractions per week, was given with concurrent interferon α-2b at 10 MU/m 2 subcutaneously 3 times per week on days alternating with radiation. Thereafter, maintenance interferon was continued for a total of 1 year of treatment. A total of 29 patients were enrolled between August 1997 and March 2000. The maximum (worst) grade of acute nonhematologic toxicity during concurrent treatment was grade 3 skin toxicity noted in 2 patients (9%). Late effects were limited but included 1 patient with a grade 4 brachial plexus injury. Of the evaluable patients, after a median follow-up of 80 months among surviving patients, the probability of regional control was 78% at 12 months, the median relapse-free survival was 20 months, and the median overall survival was 35 months.
Although these prospective data may be encouraging, care must be exercised when combining radiotherapy with interferon, especially when using high dose per fraction radiation. Given that a few high-grade late effects have been observed in some concurrent radiation/interferon reports, off protocol we prefer to administer the 1-month intravenous induction phase of interferon therapy, slightly delaying the subcutaneous maintenance phase until after the completion of conventionally fractionated regional basin irradiation.
Radiotherapy with Concurrent Chemotherapy
There are few data regarding the value of concurrent radiation therapy and chemotherapy in the treatment of melanoma. This is partly because of concerns about adding chemotherapy-related toxicity in a cohort for whom the treatment goal is usually palliative. In addition, the intrinsic value of cytotoxic chemotherapy when used without radiation in melanoma is limited. Nonetheless, there is some evidence that chemotherapy causes sensitization of melanoma cells to treatments with radiation. When using concurrent chemotherapy, radiation doses should be limited to no greater than 2 Gy per fraction for standard fractionated treatments. At our institution, we consider the use of radiation therapy with concurrent temozolomide in patients with unresectable melanoma, gross residual melanoma after resection, or very heavy microscopic burden. The rationale for adding temozolomide stems from evidence supporting its use in melanoma, its ease of administration (oral), and its putative radiosensitizing properties with reasonably well documented safety. We deliver between 40 and 70 Gy with conventional fractionation, depending on the intent (ie, palliative vs adjuvant vs definitive), while respecting the tolerances to radiation of surrounding normal structures.
Radiotherapy with Hyperthermia
Hyperthermia in the temperature range from 40 to 45°C is cytotoxic to melanoma cells. In addition, cell killing by radiation therapy is enhanced with the use of heat. In one randomized trial, patients received hyperthermia with radiation or radiation alone for metastatic or recurrent melanoma. Three fractions of 8 or 9 Gy were delivered in an 8-day period with hyperthermia (43°C for 60 minutes). At 2 years, tumor control rates were 48% in the hyperthermia group compared with 28% in the radiation alone group ( P = .008). In another randomized trial, including patients with melanoma as well as other tumor types, the benefit of adding hyperthermia was only noted in lesions of less than 3 cm, and the results were less conclusive. More recently a randomized trial with rigorous thermal dosimetry has been described. A variety of superficial tumors less than 3 cm in depth were included with melanoma, the latter representing around 10% of the patients. Tumors were irradiated and patients were randomized to hyperthermia only if their lesions were deemed appropriate to receive hyperthermia. Local control at death or last follow-up was observed in 48% of the heated tumors versus 25% of the tumors not receiving heat ( P = .02). This trial was encouraging because it showed that thermal dose could be prospectively prescribed, delivered, and correlated with outcome. However, at present, radiation therapy combined with hyperthermia is rarely used in clinical practice because of the limited availability of this technology and difficulties in controlling temperature at the treatment site.
Radiotherapy Dose and Fractionation
There are 2 distinct philosophies regarding dose and fractionation in the treatment of melanoma. Some centers have reported excellent disease control with hypofractionated radiotherapy (dose per fraction >2 Gy per treatment per day, typically, 30 Gy in 5 fractions in the adjuvant setting). The rationale for this approach includes the ability to complete the treatment course quickly (generally <3 weeks) and in vitro and in vivo data suggesting an advantage of high dose per fraction radiation in radioresistant melanoma cells. Those who prescribe standard fractionation in the adjuvant setting (2 Gy per day) are not convinced there is a difference in efficacy between the 2 treatment schemes, and cite the ability to increase the total dose to 60 Gy or more and the reduced risk of late side effects.
The only prospective comparison of fractionation was reported by the Radiation Therapy Oncology Group in the palliative setting. This trial, with a primary endpoint of response in the treated site, randomized 137 patients with measureable metastatic melanoma to 4 fractions at 8 Gy once weekly for 21 days versus 20 fractions at 2.5 Gy, 5 days a week. Response rates were nearly identical in the 2 arms (complete response rates of 24% vs 23% and partial response rates of 36% vs 34%). Survival was not reported. Grade 3 late skin morbidity was defined as marked atrophy or gross telangiectasia, and grade 4 consisted of ulceration. Three grade 4 toxicities and 3 grade 3 toxicities were noted in the 4 times 8 Gy arm compared with only 4 grade 3 toxicities in the 20 times 2.5 Gy arm. With short follow-up and with both arms receiving nonstandard treatment schemes, toxicity data from this trial are difficult to interpret. For palliation in the metastatic setting, 30 to 36 Gy at 3 Gy per fraction is usually given, as discussed later.
When using hypofractionation, it is critically important that hot spots are kept to a minimum and that the spinal cord, brain, and bowel maximum dose are kept to less than 24 Gy total. In addition, using electrons when possible may be prudent to limit the radiotherapy dose at depth and thus decrease the probability of treatment complications. Although each case is individualized, in the adjuvant setting we prefer conventionally fractionated therapy at 2 Gy per fraction, delivering a total dose between 54 Gy and 66 Gy depending on the anatomic site. We are especially wary of treating large volumes of normal tissue with large dose per fraction radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree