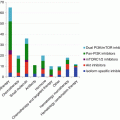
Drug
Target
Stage of development and indications
Company
mTOR
Sirolimus (rapamycin)
mTOR
Approved for renal transplant, phase II/III trials for MM, NHL
Pfizer
Temsirolimus (CCI779)
mTOR
Approved for RCC and MCL, phase II/III trials for MM, NHL
Pfizer
Everolimus (RAD001)
mTOR
Approved for advanced RCC, phase II/III for lymphomas
Novartis
Ridaforolimus (AP23573)
mTOR
Phase III trials for relapsed hematological malignancies
Ariad/Merck
AZD8055
mTOR
Phase I/II for lymphomas
AstraZeneca
OSI-027
mTOR
Phase I/II for lymphomas
OSI pharma
Panobinostat (LBH-589)
mTOR, HDAC
Approved for MM. Phase II/III for hematological malignancies
Novartis
INK128(MLN0128)
mTOR
Phase I for MM/WM
Millennium
CC-115
mTOR, DNA-PK
Phase I for advanced hematological malignancies
Celgene
PI3K
AMG 319
PI3K P110δ
Phase I CLL or NHL
Amgen
BAY80-6946
Pan-PI3K
Phase II NHL
Bayer
BKM120
Pan-PI3K
Phase I/II advanced NHL
Novartis
BYL719
PI3K P110ɑ
Phase Ib/II advanced MM, leukemia, MDS
Novartis
CUDC-907
PI3K, HDAC
Phase I advanced MM or lymphoma
Curis
Dactolisib (BEZ235)
mTOR, PI3K
Phase I advanced leukemia
Novartis
Duvelisib (IPI-145)
PI3K P110ɣ/δ
Orphan drug approval for CLL/SLL. Phase I/II/III for hematological malignancies
Infinity Pharmaceuticals
GDC-0980
mTOR, PI3K
Phase I advanced NHL
Genentech
GSK2636771
PI3K p110ɓ
Phase I/IIia advanced lymphoma
GlaxoSmithKline
Idelalisib (GS-1101, CAL-101)
PI3K P110δ
Approved for CLL, SLL, and follicular lymphoma. Phase II/III ongoing for hematological malignancy.
Gilead
INCB040093
PI3K P110δ
Phase I advanced B-cell malignancies
Incyte
SAR260301
PI3K p110ɓ
Phase I advanced lymphomas
Sanofi
TGR 1202
PI3K P110δ
Phase I advanced hematological malignancies
TG Therapeutics
XL147 (SAR245408)
mTOR, PI3K
Phase I advanced lymphoma
Sanofi
XL765 (SAR245409)
mTOR, PI3K
Phase I/II CLL or NHL
Sanofi
AKT
Perifosine (KRX-0401)
Akt
Orphan drug approval for neuroblastoma, MM phase III halted
AEterna Zentaris
MK2206
Akt
Phase I/II advanced hematological malignancy
Merck
Afuresertib (GSK2110183)
Akt
Phase I/II advanced hematological malignancy
Novartis AG
7.2 The Role of PI3K/AKT/mTOR Signaling in Normal Hematopoiesis and Leukemogenesis
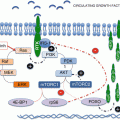
In normal hematopoiesis, all blood cellular components are formed in a tightly regulated process involving a delicate balance between hematopoietic stem cells, bone marrow microenvironment, and signaling cytokines. Given the high turnover of mature blood cells, the hematopoietic system needs to rapidly respond to daily demands as well as physiological stressors such as hemorrhage and infection in order to preserve a steady state. Cytokines, such as stem cell factor (SCF) and interleukins (IL), are a family of extracellular ligands that can communicate and initiate biological reactions between many different cell types. The PI3K/AKT/mTOR signaling pathway is a key regulator of this process, controlling cellular proliferation, differentiation, survival, motility, autophagy, and metabolism [1].
Most adult hematopoietic stem cells (HSCs) stay in a quiescent state or the G0 phase of the cell cycle in order to maintain HSC functions and protect themselves from environmental stressors [2]. However, the PI3K/AKT/mTOR plays a key role in HSC activation and differentiation. Recent studies have shown that mouse HSCs reenter the cell cycle by upregulating AKT and that treatment of HSCs with interferon-alpha (INFa) increases AKT1 phosphorylation leading to active cell cycling [3, 4]. Chronic INFa exposure, as expected, then impairs HSC ability to repopulate. Interestingly, conditional deletion of PTEN (phosphatase and tensin homolog, a tumor suppressor gene), a negative regulator of AKT, results in initial expansion of murine HSC population from increased cell cycling followed by exhaustion of HSC populations [5]. Rapamycin is able to revert the phenotype of the PTEN knockout HSCs, suggesting that mTOR signaling is responsible for increased cycling and subsequent loss of HSC maintenance [6]. Many other studies support the importance of mTOR signaling in HSC proliferation and pluripotency [7–9].
Differentiation occurs when pluripotent HSCs become progenitor cells with restricted lineages and eventually differentiate into specific types of cells. Varying levels of PI3K and AKT activation play an active role in lineage choice decisions [10]. This pathway has been delineated to show that AKT controls downstream phosphorylation of effector proteins that are in constant cross talk with other signaling pathways in order to control hematopoietic progenitor differentiation [10, 11].
Erythropoiesis, or the production of red blood cells, is tightly regulated by erythropoietin (EPO) and SCF. EPO and SCF exert their actions through the JAK/STAT5, RAS/RAF/MEK/ERK, and the PI3K/AKT/mTOR pathways. EPO and SCF signal through the mTOR pathway to regulate cell cycle and to control differentiation. For instance, SCF signaling through PI3K delays erythroblast differentiation, and PI3K inhibition increases it [12]. Another mechanism involves AKT regulation of FOXO3 (transcription factor Forkhead box O). In normal erythroid differentiation, FOXO3 activity controls reactive oxygen species (ROS) levels through transcription of antioxidant enzymes. Moreover, FOXO3 activity itself is required for erythroblast cell cycling. In a FOXO3-deficient model, an increased ROS level activates AKT signaling which then decreases erythroblast maturation by influences on cellular metabolic activities. mTOR signaling inhibition alleviates abnormal maturation of FOXO3-deficient erythroblasts and leads to increased erythropoiesis [13]. PI3K/AKT also directly phosphorylate and activate transcription factors important in EPO signaling including GATA-1 (globin transcription factor-1), a key regulator of erythroid differentiation, and p70S6K (downstream kinase of PI3K that induces protein synthesis when phosphorylated) and many other genes important in the regulatory role between EPO, SCF, and erythroblasts [14–16].
In megakaryopoiesis, HSC undergo lineage commitment to become megakaryoblasts and then mature megakaryocytes (MK) that produce platelets. Thrombopoietin (TPO), produced by the liver and kidney, stimulates production and differentiation of MK progenitor cells by activating downstream signaling pathways similar to those involved in erythropoiesis. TPO specifically has been shown to stimulate phosphorylation of AKT in megakaryoblasts to protect cells from apoptosis [17]. In vivo studies have shown that blocking this pathway with rapamycin resulted in MKs that are diminished in size and number, delayed in maturation, and produce less platelets [18]. While PI3K/AKT/mTOR signaling is necessary for TPO induced MK proliferation, it is not sufficient and other pathways play a critical role in the regulation of cell cycle [19].
The PI3K/AKT/mTOR pathway is also implicated in leukemogenesis, or the transformation of normal HSC or progenitor cells into leukemic stem cells (LSC). Leukemia is thought to occur as the result of multiple genetic mutations or “hits” resulting in dysregulated growth and enhanced cell survival. A constitutively activated mTOR pathway can trigger dysregulated growth especially in the setting of other mutations that promote cellular survival and the transformation of HSC to LSC [20]. In mouse models, hematopoietic cells expressing an activated catalytic subunit (p110a) of PI3K transformed into a leukemia-like disease characterized by anemia and neoplastic infiltration of the bone marrow [21]. Similar results were seen in murine bone marrow transplant models where constitutively active AKT signaling resulted in myeloproliferative disease (MPD), T-cell lymphoma, or AML. Analysis of the HSCs in bone marrow of these transplanted mice revealed expansion and increased cycling as well as impaired engraftment [22]. However, other experiments show that mTOR pathway activation leads to HSC exhaustion rather than leukemogenesis, and thus other contributing pathways intersecting with PI3K/AKT/mTOR act as the switch between the two processes [23–25]. The role of PI3K/AKT/mTOR signaling in specific hematological malignancies will be discussed in more detail in later sections.
Although the PI3K/AKT/mTOR complex does play critical roles in both normal hematopoiesis and leukemogenesis, it is difficult to fully delineate all of its roles as it controls numerous molecular targets depending on the cellular context. In vitro studies thus far have added to the complexity of the issue as knockout models may exhibit different phenotypes from drug-induced inhibition. Further research is needed to characterize PI3K/AKT/mTOR signaling in specific hematological disorders to direct effective therapy targeting this pathway.
7.3 Acute Myeloid Leukemia
Acute myeloid leukemia (AML), the most common acute leukemia affecting adults, is a malignant disease characterized by the clonal proliferation of immature myeloid cell and interference of normal hematopoiesis. Despite advances in treatment, AML remains challenging to treat and a large percentage of patients relapse. Patients that do particularly poorly are those over the age of 60, patients with poor risk cytogenetics or genetic mutations such as Fms-like tyrosine kinase 3 (FLT3), and patients with AML arising out of antecedent myelodysplastic syndrome. In this high-risk group, the conventional chemotherapy regimen consisting of cytarabine and anthracycline has only limited efficacy. Therefore, there is increasing focus on developing targeted therapy of key signaling pathways either alone or in combination that may result in less toxic and more effective therapy.
Previous studies have shown that the PI3K/mTOR/AKT pathway is upregulated in about 50–80 % of the AML cases, with most of the cases characterized by constitutively phosphorylated AKT [26, 27]. Activation of PI3K/mTOR/AKT survival pathways frequently occurs as the result of activating mutations in kinase receptors such as FLT3 and c-KIT [28]. Multiple downstream effectors and abnormal secretion of autocrine/paracrine such as insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) are also activated as a result of this signaling pathway [29, 30]. Indeed, preclinical data has shown that the PI3K/mTOR/AKT pathway is necessary for survival of AML blasts and that targeting this pathway with LY294002, a PI3K inhibitor, and rapalogs have resulted in decreased AML cell growth [31, 32].
Consistent with this preclinical data, several clinical studies have shown promising results with mTOR/PI3K inhibitors. However, given the molecular complexity of AML, early phase trials evaluating rapalogs as single agents did not show significant activity in AML [33, 34]. Subsequently, the focus shifted toward combination therapy. In a phase I study of sirolimus combined with MEC (mitoxantrone, etoposide, cytarabine) in patients with relapsed, refractory, or untreated secondary AML, 22 % of the patients achieved a partial response (PR) or a complete response (CR), though the synergistic mechanism was not clearly delineated or confirmed [35]. In a phase Ib study of everolimus with low-dose cytarabine (LDAC) in 24 untreated elderly (median age 74) AML patients, the 24 patients with a median age of 74 had an overall response rate (ORR) of 34 % (13 % CR, 4 % CR with incomplete blood count recovery [CRi], 17 % PR) [36]. In the stratified matched analysis against LDAC alone, LDAC with everolimus had superior median survival in poor risk patients and no statistical difference in outcome compared to patients treated with intensive chemotherapy.
Most recently, in a phase Ib study, everolimus was combined with conventional induction chemotherapy (7 + 3 cytarabine and daunorubicin) in 28 patients under 65 years of age at first relapse following prior chemotherapy or allotransplantation [37]. Encouragingly, 68 % of patients (19 patients) achieved a CR although 14 patients had to receive a second induction course at day 15. At the higher dose of everolimus, the CR rate reached 85 %, and 8 total patients in the study were able to proceed to allotransplantation. These results compare favorably to CR rates with conventional chemotherapy in relapsed patients (late relapse: CR range, 4–83 %; early relapse, range 18–41 %). Other promising strategies in AML include combining mTOR inhibitors with hypomethylating agents such as azacitidine and decitabine [38, 39]. Lastly novel inhibitors of AKT are being evaluated in the clinical and preclinical settings in AML [40, 41].
7.4 Myelodysplastic Syndrome
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders characterized by ineffective hematopoiesis and variable risk of transformation into AML. The AKT/mTOR pathway is also constitutively active in many cases of MDS, with high levels of phosphorylated AKT found in the high-risk MDS patients but not in normal bone marrow or low-risk MDS patients [42]. Preclinical studies have shown that mTOR effector molecules such as 4E-BP1 and p70S6K are involved in hematopoietic cell proliferation. Consistent with this finding, rapamycin decreased the in vitro clonogenic activity of high-risk MDS cells [43]. Clinical data supporting these results include a phase I/II study in patients with relapsed or refractory hematological malignancies that received single agent everolimus, an oral mTOR inhibitor. In this study of 27 patients, 5 patients had MDS, two of whom were able to achieve some improvements in platelet count and the treatment was well tolerated [33]. Platzbecker et al. [44] reported a pilot study of 19 MDS patients (3 of whom had received prior therapy) who received sirolimus orally. In this study three patients (one with refractory anemia with excess blasts [RAEB]-2, one with RAEB-1, and one with refractory cytopenia with multilineage dysplasia) showed either major (1× platelet, 1× neutrophil) or a minor (1 × erythroid, 2 × platelet) hematological responses according to International Working Group criteria. mTOR and AKT inhibitors in combination with hypomethylating agents or chemotherapy are currently undergoing investigation for use in MDS patients.
7.5 Chronic Myelogenous Leukemia
Chronic myelogenous leukemia (CML) is characterized by unregulated growth of myeloid precursors in the bone marrow and proliferation of mature granulocytes (neutrophils, eosinophils, and basophils) in the peripheral blood. CML became a model for effective molecular targeted therapy when the discovery of the oncogenic fusion protein, BCR-ABL, produced by the t(9, 22) translocation, led to the development of tyrosine kinase inhibitors (TKI) such as imatinib, dasatinib, and nilotinib. Despite the success of these agents, resistance can develop due to the emergence of mutations in the BCR-ABL kinase domain, such as the T315I mutation caused by an amino acid substitution at position 315 in BCR- ABL1, from a threonine (T) to an isoleucine (I), hindering the binding of TKIs. Other BCR-ABL mutations that confer varying degrees of resistance to TKIs are also emerging making targeting of pathways downstream of BCR-ABL more attractive.
The mTOR/PI3K pathway is a major effector signaling pathway downstream from BCR-ABL that in turn triggers the expression of vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1 alpha (HIF-1α), both of which result in increased angiogenesis in CML [45]. In one small pilot study, Sillaber et al. [46] treated six patients with imatinib-resistant CML in hematological relapse (leukocytes >20,000 μL−1) with rapamycin. Two patients had a major leukocyte response with a decrease in WCC to less than 10,000 μL−1 with minor transient responses seen in two other patients. Responding patients also had decrease in VEGF mRNA levels in circulating leukemic cells with in vivo inhibition of imatinib-resistant (including T315I mutated) cells of BCR-ABL. Another preclinical study reported that the dual mTORC1/2 inhibitor, OSI-0217, induced apoptosis in CML progenitors including T315I mutant cells [47]. PI3K inhibition is also being studied in combination with TKIs and has displayed favorable results in preclinical studies. In one study, LY294002, a potent PI3K inhibitor, was able to restore nilotinib-induced apoptosis of CML stem cells that were previously refractory to nilotinib due to activation of the SCF survival pathway [48]. NVP-BEZ235, a dual PI3K and mTORC 1/2 inhibitor, has also been shown to be effective in enhancing cytotoxicity in CML stem cells and progenitor with different TKIs [49]. CML stem cells are thought to be generally resistant to TKI making cure with TKI therapy alone unlikely. These studies suggest that combining TKIs with inhibitors of the mTOR/PI3K pathway may effectively target CML stem cells and offer the possibility of cure in addition to overcoming resistance.
7.6 Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is a lymphoid malignancy resulting from clonal proliferation of early B- and T-cell precursors and represents the most common pediatric malignancy. While children with ALL have an excellent outcome, adults with ALL tend to be more refractory to chemotherapy and relapse frequently. Recent improvements in treatment have been made such as the addition of TKIs to traditional chemotherapy in Philadelphia chromosome positive (Ph+) ALL [50]. Activation of PI3K/mTOR/AKT occurs frequently in ALL by various mechanisms such as inactivation of PTEN in precursor T-cell ALL and subsequent activation of AKT [51] or by direct activation by BCR-ABL in Ph + pre-B-ALL [52].
T-cell ALL represents about 25 % of adult ALL and has a poor prognosis due to high frequency of relapses despite good response to initial chemotherapy [53]. Due to the high incidence of PTEN mutations, mTOR is an attractive target and has been tested in combination with conventional chemotherapy. In particular, rapalogs have shown to be synergistic in preclinical models in combination with chemotherapies such as dexamethasone [54], methotrexate [55], doxorubicin [56], cyclophosphamide, and vincristine [57]. Dual PI3K/mTOR inhibition with NVP-BEZ235 also has activity against T-ALL cell lines and patient-derived blasts [58]. In a phase I clinical trial of pediatric patients with relapsed/refractory ALL, sirolimus was well tolerated with no dose limiting toxicity and maintained stable disease in three out of seven patients [59]. More recently, an open label single center phase I/II study of everolimus in combination with HyperCVAD in patients with relapsed/refractory ALL was completed that included 24 patients with an average of 2 prior treatments (median age of 25) [60]. Grade III mucositis was the major dose limiting toxicity. The ORR was 33 % with 6 CRs, 1 CR without platelet recovery (CRp), and 1 CR without recovery of counts. Additionally, 7 of 11 patients treated in first salvage achieved CR/CRp (64 %). The T-ALL group was more heavily pretreated with a median of four prior therapies compared to B-ALL patients with a median of one prior therapy, but the median OS was similar between the T-ALL and B-ALL group (23 weeks).
B-ALL is the more common subtype of ALL and can often feature Ph + that express the fusion protein BCR-ABL that in turn activates the PI3K/mTOR/AKT survival pathway. As in CML, the fusion protein BCR-ABL can be effectively blocked by TKIs such as imatinib. Consistent with similar studies in CML, preclinical data have shown that rapamycin can restore imatinib sensitivity in TKI-resistant Ph + ALL cell lines [61]. Similarly, BEZ235, a dual PI3K/mTOR inhibitor was also able to overcome nilotinib resistance in Ph + B-ALL [62]. In Philadelphia chromosome negative (Ph-) B-ALL, the potential role of PI3K/mTOR/AKT pathway is less well understood. However, this should not exclude evaluation of inhibition of the pathway as a potential therapeutic target. In fact, preclinical data show that dual inhibitors of PI3K/mTOR, NVP-BGT226 and NVP-BEZ235, have antiproliferative and proapoptotic effects in Ph-B-ALL cell lines suggesting that PI3K/mTOR inhibitors may be useful in all subtypes of ALL [63].
7.7 Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is characterized by the progressive accumulation of monoclonal, immunologically incompetent mature lymphocytes in the blood, bone marrow, and lymphatic tissue. CLL and small lymphocytic lymphoma (SLL) are considered different manifestation of the same disease and together they comprise the most common adult leukemia in Western countries. The B-cell receptor (BCR) pathway is critically important in the pathogenesis of CLL [64, 65]. Upon activation, BCR activates LYN and SYK kinases, which then stimulate several downstream mediators including the PI3K/mTOR/AKT and bruton tyrosine kinase (BTK) pathways essential for B-cell survival, proliferation, and differentiation. Consistent with the critical role of mTOR in CLL, rapamycin can induce a G1 cell cycle arrest by reducing the expression of several key regulators of cell cycle progression including cyclin D3, cyclin E, and cyclin A [66]. These findings prompted a small pilot study of single agent everolimus in seven patients with advanced, relapsed B-CLL. However, everolimus was poorly tolerated in these patients and the trial was stopped early due to toxicity mainly from immunosuppression-related infections [67]. Prior to the termination of study, one patient had PR while three patients had stable disease. Cyclin E expression decrease was noted in responding patients suggesting that everolimus induced cell cycle inhibition. A phase II study of single agent everolimus in recurrent/refractory CLL followed in which 4 of 22 patients achieved PR (18 %) [68]. Interestingly, 8 patients (36 %) had an increase in absolute lymphocyte count with a decrease in lymphadenopathies, suggesting that everolimus mobilizes CLL cells into peripheral circulation. However, the immunosuppressive effects of everolimus in this sensitive patient population were again notable. Five patients had serious infections and there were two infection-related deaths, suggesting that careful antimicrobial prophylaxis will be necessary in any future studies of mTOR inhibitors in CLL.
While mTOR inhibition in CLL may be problematic, PI3K inhibition in CLL has been shown to be quite effective. In 2014, the United States Food and Drug Administration (FDA) approved idelalisib, a selective PI3Kδ inhibitor, for the treatment of CLL/SLL. The approval came after completion of a multicenter, randomized, double-blinded phase III study in 220 patients with relapsed CLL who were less able to undergo chemotherapy The trial demonstrated that idelalisib in combination with the CD 20 monoclonal antibody rituximab significantly improved progression free survival (PFS), RR, and OS compared to rituximab alone [69]. The ORR was 81 % in idelalisib and rituximab group compared to 13 % in the placebo and rituximab group, and the OS at 12 months was 92 % versus 80 % in the idelalisib and placebo arms, respectively. The benefit of idelalisib was seen in all subgroups including the high-risk patients with either a deletion of or mutation in the P53 tumor suppressor gene. As seen with everolimus, there appears to be a release of CLL cells from the lymph node and bone marrow microenvironments into the circulation. This release of CLL cells does cause a transient lymphocytosis but coadministration of rituximab with idelalisib appears to mitigate this to some degree. In 2013, duvelisib, a dual PI3Kδ and PI3Kγ inhibitor with activity against AKT, received FDA orphan drug status for the treatment of CLL after phase II/III studies showed single agent activity in CLL [70]. Duvelisib is now being compared to another CD20 monoclonal antibody, ofatumumab, in a phase III trial in refractory/relapsed CLL [71]. Several other PI3Kδ-selective, pan-PI3K, and PI3K/mTOR dual inhibitors are also currently being studied in active clinical trials in CLL and indolent lymphoma patients.
A significant new breakthrough in the treatment of CLL has been successful inhibition of the Bruton’s tyrosine kinase (BTK) pathway. As previously discussed, the BCR forms the BCR signaling complex along with LYN and SYK, which then directly links PI3K and BTK [65]. PI3K also phosphorylates PIP3, a phospholipid kinase responsible for activating not only the downstream AKT but also the separate pathway of BTK [72, 73]. BTK is a cytoplasmic tyrosine kinase and it is expressed in all hematopoietic cells. The loss of function of BTK, especially in B cells, inhibits B-cell maturation and causes the primary immunodeficiency disease, X-linked agammaglobulinemia (Bruton’s agammaglobulinemia) [74]. Ibrutinib, previously called PCI-32765, is the first human BTK inhibitor and binds irreversibly to the BTK kinase domain [75]. Preclinical data showed that ibrutinib was able to induce CLL cell apoptosis, inhibit proliferation and chemotaxis, and downregulate BCR-dependent chemokines [76–78]. A phase 1b/2 multicenter study of ibrutinib in 85 patients with high-risk relapsed or refractory CLL/SLL demonstrated an ORR of 71 % (2 CR and 58 PR) with an additional 15 patients (18 %) having a PR with lymphocytosis [79]. Consistent with the critical role of the BCR signaling in homing CLL cells to the bone marrow and lymph nodes, ibrutinib treatment is typically associated with a transient lymphocytosis. Similar to idelalisib, responses were independent of risk factors including advance stage disease, number of previous treatments, or presence of 17p deletion. This study led to an accelerated expanded approval of ibrutinib for CLL in February 2014. Another phase II study followed with encouraging safety and efficacy data showing 40 high-risk CLL patients with 95 % (35 patients) ORR and 78 % 18 months PFS [80]. Finally, a randomized phase III study showed that ibrutinib compared favorably to ofatumumab in patients with relapsed CLL with an improved PFS and OS as well as significantly higher ORR (42.6 % vs. 4.1 %) [79].
7.8 Multiple Myeloma
Multiple myeloma (MM) is characterized by neoplastic proliferation of plasma cells that frequently produce monoclonal immunoglobulins. Novel agents including the immunomodulatory drugs (IMiDs) such as thalidomide and lenalidomide as well as proteasome inhibitors such as bortezomib have greatly improved OS in patients with MM, though the disease remains mostly incurable [81]. Preclinical studies have shown that the PI3K/AKT/mTOR signaling cascade is upregulated in a significant portion of MM patients due to the malignant plasma cell interaction with the microenvironment [82, 83]. In particular the activity of the key MM pro-survival factor, IL-6, is mediated through PI3K/AKT/mTOR. Immunohistochemistry of bone marrow biopsies of patients with MM also demonstrated activated AKT staining specifically on malignant plasma cells and no staining on the nonmalignant hematopoietic stem cells [84].
There have been several early phase clinical trials evaluating mTOR inhibitors in MM. As a single agent, mTOR inhibition with temsirolimus had minimal activity, but single agent everolimus was very active with 10 out of 15 evaluable relapsed/refractory MM patients responding [85, 86]. A phase I/II study showed that temsirolimus in combination with bortezomib had clinical activity in heavily pretreated MM patients [87]. Thirty-three percent of patients achieved a PR but the treatment was complicated by cases of grade III–IV cytopenias. Most recently, everolimus was also evaluated in a phase I study in combination with lenalidomide in 26 patients with heavily pretreated relapsed/refractory MM [88]. Although this treatment was associated with notable toxicity including fatigue, diarrhea, and cytopenias, it was also found to be very active with an ORR of 65 % (1 CR, 4 PR, and 10 minimal response [MR]).
AKT inhibition has also been evaluated in multiple myeloma. Perifosine is an orally active dual PI3K and AKT inhibitor that is being developed for cancer treatment and its initial testing were promising. It has been studied in combination with bortezomib and dexamethasone in patients with relapsed/refractory MM who were previously treated with bortezomib [89]. Perifosine appeared to resensitize MM patients to bortezomib as evidenced by the fact that 32 % of bortezomib-refractory patients responded to the combination. Therapy was relatively well tolerated with no grade 4 toxicities. Perifosine has also been studied in combination with lenalidomide and dexamethasone in a phase I study of relapsed/refractory MM showing an ORR of 73 % (MR or better) with tolerable toxicity which was mostly hematological [90]. Interestingly response correlated with active AKT signaling as evidenced by immunohistochemical p-AKT staining on pretreatment bone marrow biopsies. Unfortunately, Aeterna Zentaris has discontinued the ongoing phase 3 testing of perifosine compared to placebo when combined with bortezomib in MM following Data Safety Monitoring Board (“DSMB”) report recommending that it was “highly unlikely the study would achieve a significant primary end point, progression free survival” [91]. Afuresertib is another orally active AKT inhibitor that has shown some activity in MM (18 % ORR, MR, or better) [92]. Lastly PI3K inhibition is also currently under investigation in MM and is showing promising results in the preclinical setting [93].
7.9 Lymphoma
Lymphoma represents a heterogeneous group of clonal tumors arising from the malignant transformation of mature or immature lymphocytes. It is the most common hematologic malignancy, but the clinical presentation and outcome are highly varied, which reflects perturbations in many different molecular pathways leading to malignant transformation. Among these signaling abnormalities, the activation of the PI3K/AKT/mTOR pathway has been well documented in various types of lymphoma. For example, in diffuse large B-cell lymphoma (DLBCL), an aggressive mature B-cell non-Hodgkin’s lymphoma (NHL), constitutive activation of PI3K/AKT/mTOR has been associated with loss of PTEN activity in the germinal center subtype [94]. Moreover, the activation of the B-cell receptor (BCR), a critical signaling pathway for B-cell survival, leads to downstream activation of PI3K [95]. The PI3K/AKT/mTOR pathway has also been extensively evaluated in mantle cell lymphoma (MCL), an aggressive mature B-cell NHL characterized by the t(11;14) translocation resulting in overexpression of cyclin D1. Finally, it has been shown that 4EBP1, a major downstream effector molecule of mTORC1, promotes mRNA translation of many cell cycling genes such as cyclin D1 [96].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree