Fig. 9.1
Overall cytogenetic and molecular response rates of chronic phase CML to ponatinib within the PACE trial [14]. MCyR states for major cytogenetic response, CCyR for complete cytogenetic response, MMR for major molecular response, MR4 for molecular response 4 logs, MR4.5 for molecular response 4.5 logs, R/I for resistant/intolerant
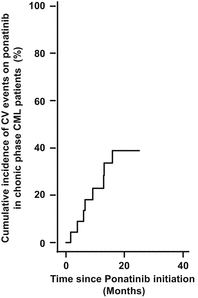
Fig. 9.2
Cumulative incidence of overall cardiovascular events on ponatinib in chronic phase CML patients as defined in [15]. CV states for cardiovascular
9.2.2 Ponatinib in Advanced Phases CML and Ph + ALL Patients
Ponatinib retains a significant activity in patients in accelerated phase at enrolment within the PACE trial [4, 14] with 59 % of living patients after 36 months of median follow-up [14]. Conversely, the activity of this compound as monotherapy in blast crisis patients and refractory Ph+ ALL remains unusual with catastrophic overall survival after a median follow-up of 36 months: 9 % and 16 %, respectively [14]. However, this compound can be useful as a therapeutic bridge or a debulking agent prepared for allogeneic stem cell transplantation.
9.3 Omacetaxine (OMA)
OMA (Synribo®, Teva) is a protein inhibitor synthesis without any tyrosine kinase activity first tested in solid tumors. Before the TKI era, OMA – formerly known as homoharringtonine – was then used as second-line therapy for chronic phase patients failing interferon-α [17, 18]. The demonstration that OMA retains activity on leukemic stem cells in murine models (90 % of the leukemic stem cells (LSCs) die after in vitro OMA exposure versus 9 % after imatinib) promoted the revival of this almost forgotten compound. Recently, it became a suitable option for the treatment of resistant CML in the CGX-635-CML-203 trial with 12.8 % of major cytogenetic responses (MCyR) and 69 % of major hematologic responses (MaHR), for chronic phase patients [19]. Interestingly, OMA exerts a specific activity on ABL mutated clones – particularly T315I – through an unknown mechanism and is able to retard or abrogate disease progression in such patients and allow TKI rechallenge (Fig. 9.3.) [20, 21].
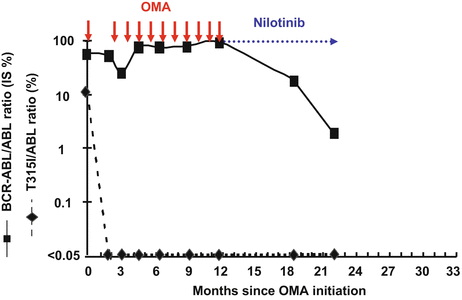

Fig. 9.3
Overall molecular and T315I mutated transcript response rates of a chronic phase T315I+ CML patient treated with several courses of OMA (top arrows) followed by a nilotinib rechallenge (dashed arrow). Published in part in [20]. OMA states for omacetaxine mepesuccinate
9.3.1 Omacetaxine in Chronic Phase CML Patients
In 1995, O’Brien et al. demonstrated some activity in late chronic phase CML patients as monotherapy with a daily dose of 2.5 mg/m2 for 14 days (CHR = 72 %, overall cytogenetic response = 31 %, CCyR = 7 %, MCyR = 15 %) [17]. The same authors went on exploring the combination of OMA + IFN-α for early chronic phase, initially as a sequential schedule: 90 patients with 2.5 mg/m2 daily as a continuous infusion for 14 days, followed by maintenance for 7 days a month. Overall cytogenetic response rates to OMA were 66 %, twice the response rates in late chronic phase with interferon-α alone, and CCyR rates were 23 % (see a summary of the responses described in the literature in Table 9.1). When administrated simultaneously, a further improvement in the CHR rates, overall cytogenetic response rates, and MCyR rates was seen with 84 %, 69 %, and 52 %, respectively, in patients with early chronic phase and 80 %, 50 %, and 40 %, respectively, in late chronic phase. The estimated 2-year survival rate was 90 % (Table 9.1) [22]. In 2002, preliminary in vitro results suggested that imatinib resistance was not generally associated with resistance to Ara-C, daunorubicin, and OMA and may be synergistic to imatinib [23]. Furthermore, because OMA retains some activity on Ph+ cells via different cellular pathways than TKI, D. Marin et al. hypothesized that OMA would reduce the level of residual disease in patients with CML in suboptimal cytogenetic response to imatinib with a molecular response observed the majority of patients [24]. In a combination sequence including imatinib + OMA, 63 % of patients reached the CCyR state (versus 20 % without imatinib) and 76 % the MCyR state (versus 46 % without imatinib). The estimated 5-year OS rate was 88 % in this study (Table 9.1) [25]. In a phase 1/2 trial, regardless of the ABL mutational status, Cortes et al. explored the safety and efficacy of OMA in first-line imatinib failure patients. All patients obtained at least a CHR and just 1 a CCyR, with undetectable BCR-ABL transcripts [26]. In our center, OMA demonstrated impressive and long-lasting (>5 years) major molecular response in interferon-α- and multi-TKI-resistant chronic phase CML patient (Fig. 9.4). Recently, data were pooled from 2 phase 2 trials conducted on 122 OMA CML patients (81 in CP, 41 in AP) regardless of the mutation status previously been treated with two or more TKIs introduced OMA as a valuable option for TKI-resistant CML: 20 % of patients in CP achieved a major MCyR (CCyR = 10 %, PCyR =10 %) with a median duration of MCyR of 18 months. The median overall survival was 34 months [26, 27].
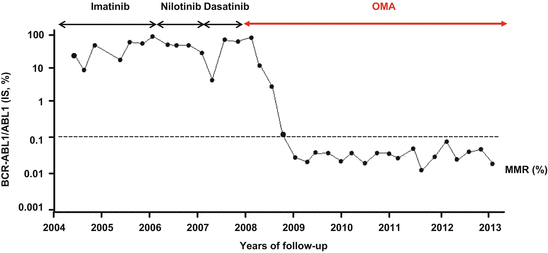
Table 9.1
Overall response rates and survival in chronic and accelerated phase CML patients receiving omacetaxine mepesuccinate (OMA) for TKI resistance
Trials | Drug | N | CHR (%) | MCyR (%) | CCyR (%) | Overall CyR (%) | OS/PFS (months) | BCR-ABL (%) |
|---|---|---|---|---|---|---|---|---|
T315I negative | ||||||||
O’Brien et al. (1995) [17] | OMA 2.5 mg/m2 | 71 | 72 | 15 | 7 | 31 | 30/NA | NA |
O’Brien et al. (1999) [41] | OMA 2.5 mg/m2 + IFN 5MUI/m2 | 90 | 92 | 44 | 23 | 66 | NR | NA |
Kantarjian et al. (2000) [42] | OMA 2.5 mg/m2 + Ara-C 2.5 mg/m2 | 105 | 72 | 15 | 5 | 32 | 55 % at 4 years | NA |
O’Brien et al. (2002) [22] | OMA 2.5 mg/m2 + IFN 5MUI/m2 | 47 | 84 | 52 | 21 | 66 | 90 % at 2 years | NA |
Cortes et al. (2013) [19] | OMA 1.25 mg/m2 | 81 | 69 | 20 | 10 | NA | 34/9.6 | NA |
Nicolini et al. (2013) (Accelerated phase) [27] | OMA 1.25 mg/m2 | 41 | 24 | 0 | 0 | 15 | 16/4.7 | NA |
T315I positive | ||||||||
Nicolini et al. (2010) [20] | OMA 1.25 mg/m2 ± nilotinib 800 mg/j | 8 | 100 | / | 37.5 | / | / | <0.1 % in 50 % of patients |
Cortes et al. (2012) [30] | OMA 1.25 mg/m2 | 62 | 77 | 23 | 16 | 44 | NR/7.7 | <0.1 % in 15 % of patients |
Case reports T315I in chronic phase CML | ||||||||
Legros et al. (2007) [21] | OMA 2.5 mg/m2 | 1 | 5.5 months | / | / | NR | Alive | <0.1 % (5.5 months) |
de Lavallade et al. (2007) [28] | OMA 1.25 mg/m2 | 1 | 5 months | / | Reached | / | Alive | 27 to 100 % |
Coude et al. (2012) [29] | OMA 1.25 mg/m2 + nilotinib 800 mg/j | 1 | / | / | Reached | / | Alive | <0.1 % (30 months) |

Fig. 9.4
Long-term molecular follow-up of an interferon-α- and unmutated multi-TKI-resistant chronic phase CML patient treated with OMA
In addition, and interestingly, OMA demonstrated some significant activity in anecdotic observations in patients with a T315I mutation as suspected by us and others [21, 28, 29]. The formal demonstration of its activity was provided by the results of a phase 2 international trial of OMA in 62 chronic phase CML patients harboring the T315I mutation [30]. Complete hematologic response was observed in 48 (77 %) patients, and the median response duration was 9.1 months. Fourteen (23 %) patients achieved MCyR including CCyR in 10 (16 %). The median progression-free survival was 7.7 months (Table 9.1).
9.3.2 Omacetaxine in Advanced Phase CML Patients
In accelerated phase resistant to TKI, OMA was able to induce 27 % of major hematologic response and 14 % any cytogenetic response (but no CCyR) for a median duration of 9 months [27], and the median overall survival was 16 months (Table 9.1). Despite poor response rates in this setting, OMA can serve as a bridge toward allogeneic stem cell transplantation, which remains the best option for long-term survival in patients with advanced phase CML (Table 9.1). In blast crisis, OMA does not show any activity in lymphoid blast crisis and very little activity in myeloid blast crisis [31].
9.4 Combination of TKI and Interferon-α
9.4.1 Imatinib and Interferon-α
Preliminary studies combining high doses of imatinib and pegylated interferon-α have demonstrated its feasibility and showed some restricted activity in this setting [32]. In the long term, imatinib alone fails to control the disease because of resistance or significant intolerance [1, 2], and because interferon-α retains activity on Ph + cells through different means that TKIs do, it was hypothesized that a combination of these two categories of compounds might be successful in the first-line setting. In this perspective, we [33] and others [34, 35] have launched trials combining a 40-kD-branched polyethylene glycol interferon-α improving its tolerability and half-life (Peg-interferon-α-2a Pegasys®, Roche, or Peg-interferon-α-2b ViraferonPeg®, Merck) and imatinib 400 mg/day in first-line patients. These trials demonstrated significant improvements in molecular responses despite increasing toxicities and limitation of progressions but no impact on survival. In one study [34], the improvement was uncertain, even after 5 years [36], due to poor compliance to Peg-interferon-α-2b. A summary of the responses and survival is presented in Table 9.2.
Table 9.2




Overall response rates and survival in chronic phase CML patients receiving a combination of Peg-interferon-α and imatinib first line
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree