Pathway alteration
Incidence
Tumor type
References
mTOR overexpression
6/9 (67 %)
pNET
Shida et al. [11]
Mutations in PTEN, TSC2, PIK3CA
10/68 (15 %)
pNET
Jiao et al. [12]
Akt activation
28/46 (61 %)
NET
Ghayouri et al. [13]
TSC2 and PTEN protein alterations
61/72 (85 %)
pNETs
Missiaglia et al. [14]
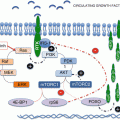
The PI3K/Akt/mTOR pathway plays an important role in cellular proliferation, growth, and metabolism. This signaling pathway is extensively detailed in another chapter and only a cursory description will be given here. The PI3K family of lipid kinase phosphorylate and the 3′-hydroxyl group of phosphoinositides are composed of three classes (I-III) with distinct lipid products, substrate specificity, and functionality. PI3K and Akt are upstream from the mTOR complexes. The activated PI3K triggers the conversion of phosphatidylinositol-4,5-diphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 then promotes the activation of Akt, also known as protein kinase B, a serine/threonine kinase and is a key regulator of PI3K and mammalian target of rapamycin (mTOR) signaling. Activated Akt stimulates mTOR complex 1 to elicit multiple cellular processes and is an important catalyst of malignant progression and chemoresistance.
mTOR is a serine/threonine kinase and is the downstream effector of the PI3K-activated signaling pathways. It promotes protein synthesis and cell growth during nutrient-rich periods and functions as a sensor of nutritional or metabolic stress during cell development [17]. mTOR regulates apoptosis, proliferation, and cell growth and also modulates mRNA-translation of proteins necessary for cell cycle progression from G1- to S-phase, including E4-binding protein (E4-BP1) and p70 kinase [18]. It represents an important break point in the proliferation and differentiation of tumor cells and is critical for regulating cell proliferation, angiogenesis, and metabolism.
There are two complexes that comprise mTOR, mTOR complex-1 (mTORC1), and mTOR complex-2 (mTORC2). mTORC1 is composed of mTOR, regulatory-associated protein of mTOR (Raptor), and target of rapamycin complex subunit LST8. mTORC1 regulates cellular transcription and translation via eukaryotic translation initiation factor 4E-binding protein-1 (4EBP-1) and ribosomal S6 kinase-1 (S6K1). mTORC2 consists of mTOR and target of rapamycin complex subunit LST8, rapamycin-insensitive companion of mTOR (rictor), and mitogen-activated protein kinase-associated protein-1. The role of mTORC2 is less well defined, but is known to directly phosphorylate Akt in the PI3K-Akt pathway [14].
Clinical syndromes appear to support the role of the PI3K/Akt/mTOR pathway in NET tumorigenesis. Inherited diseases such as multiple endocrine neoplasia type I (MEN1), tuberous sclerosis complex (TSC), neurofibromatosis type I, and von Hippel-Lindau (VHL) disease are associated with an increased incidence of PNETs. Across these syndromes, mutations in well-defined oncogenes and tumor suppressor genes (TSC2, NF1, and vHL genes) lead to constitutive activation of the PI3K/Akt/mTOR pathway. Alterations in PI3K/Akt/mTOR pathway have also been implicated in sporadic pNETs tumorigenesis justifying its exploitation as a target for rationale therapy [12, 14, 19].
Investigations of the PI3K/Akt/mTOR pathway in NETs reveal an association between its activation and cancer development. In neuroendocrine cell lines, PI3K mutations have been associated with response to mTOR inhibition [20]. Activation and phosphorylation of Akt has also been reported in a majority of neuroendocrine tumors [21, 22]. Phosphorylated Akt is a prognostic marker associated with worse outcomes in gastrointestinal NET [23]. MEN1 gene mutations, the hallmark of MEN syndromes, are associated with Akt activation [24]. These mutations have been identified in 10–35 % of foregut NETs and PNETs, both functional and nonfunctional [25–28]. Preclinical studies have also shown that mTOR and its downstream targets are overexpressed in NETs and associated with a higher proliferative index [29]. In clinical studies, expression of mTOR and its pathway components was predictive of response to temsirolimus [30].
Activation of the PI3K/Akt/mTOR pathway is likely driven by dysregulated tyrosine kinases and signaling by vascular endothelial and insulin growth factors. Studies demonstrate that receptors including PDGFR, EGFR, and c-kit are overexpressed in endocrine tumors [31, 32]. NETs and NET cell lines frequently express both IGFs and the IGF-1R receptor suggesting autocrine and/or paracrine signaling [33, 34]. IGF-1R binding leads to the direct activation of signaling cascades in the MAPK and PI3k kinase pathways [35]. The clinical benefit from somatostatin analogs in insulin growth factor secreting tumors suggests an important interplay in NET tumorigenesis and activation of PI3K/Akt/mTOR pathway [36].
Two key negative regulators of the PI3K/Akt/mTOR pathway are phosphatase and tensin homolog (PTEN) and tuberous sclerosis protein 2 (TSC2). PTEN is a tumor suppressor that negatively regulates the PI3K/Akt/mTOR pathway by converting PIP3 back to PIP2 and reversing PI3K activation. TSC2 is phosphorylated and inhibited by Akt which suppresses mTOR signaling thereby attenuating its negative regulation of the PI3K pathway [37]. Based on tissue microarray gene expression analysis, both tumor suppressor proteins were found to be downregulated in 72 primary pNET samples. Furthermore, low expression of TSC2 and PTEN was significantly associated with more aggressive tumors and with shorter disease-free and overall survival [14]. In NETs, PTEN loss or mutation promotes carcinogenesis and is associated with poor differentiation [22, 23].
Amplified angiogenesis is a distinguishing feature of well-differentiated NETs and may be associated with activation of the PI3K/Akt/mTOR pathway [38]. Activation of the PI3K pathway may also be led by the overexpression of VEGFR1 in the companion vasculature suggesting an interaction between this pathway and angiogenesis [25]. Mutations in the FLT1/VEGFR1 gene have been detected in pNET cell lines [25].
5.3 mTORC 1 Inhibitors and NETs
5.3.1 Temsirolimus
Temsirolimus (CCI-779, Torisel®, Pfizer) was the first mTOR inhibitor developed and identified to have antitumor activity [39]. After years of development, it was recently approved for the treatment of advanced renal cell carcinomas and pancreatic neuroendocrine tumors. Temsirolimus forms a complex by binding to the intracellular protein peptidylprolyl cis-trans isomerase FKBP1A (FKBP-12) that inhibits the activity of mTOR. This subsequently results in a G1-phase growth arrest, blocking its ability to phosphorylate S6K1 and the ribosomal protein S6, a reduction of HIF-1α, and VEGF expression [40]. In patients with advanced NET, a phase II study was conducted to evaluate the safety, efficacy, and pharmacodynamics of temsirolimus. Thirty-six patients with advanced and progressive NETs (21 carcinoids and 15 pNET) received weekly doses of intravenous temsirolimus. There was no difference in the objective response rates between carcinoids (4.8 %) and pNET (6.7 %). The intent-to-treat response rate for the entire cohort was 5.6 % (95 % CI 0.6–18.7 %), median TTP was 6 months, and 1-year PFS was 40.1 %. Two patients achieved partial responses (one patient with pNET and one patient with carcinoid tumor). Overall, the treatment was well tolerated with fatigue (78 %), hyperglycemia (69 %), and rash/desquamation (64 %) being the most common drug-related adverse events of all grades after a median of four cycles delivered per patient [30].
Pharmacodynamic analysis demonstrated that temsirolimus effectively inhibited the PI3K/Akt/mTOR pathway. Phosphorylation of the ribosomal protein S6 was significantly depressed (p = 0.02). Additionally, patients with an increased expression of phosphorylated Akt (p = 0.041) and a decreased expression in phosphorylated mTOR after 2 weeks of treatment were both associated with an increase in time to progression (p = 0.04 and p = 0.05, respectively). Elevated baseline levels of phosphorylated mTOR predicted a better response (p = 0.01). Even though the results of this study revealed temsirolimus value in downregulating mTOR’s downstream signaling, the authors concluded that it has limited clinical efficacy and does not support its use as monotherapy in patients with advanced NETs [30].
The limited benefit but excellent tolerability of this agent lends it to be partnered with additional agents. Preclinical studies suggest enhanced antitumor effects with temsirolimus and VEGF-targeted therapy. Therefore, a phase II study of temsirolimus in combination with bevacizumab, an anti-VEGF-A monoclonal antibody, in advanced, recurrent, or progressive pNETs (NCT01010126), was completed. Of the 56 patients eligible for response assessment, partial responses were seen in 41 % (23 of 56) patients, and 79 % of the patients (44/56) had disease stability at 6 months. Median progression-free survival was 13.2 months and overall survival was 34 months. This combination was very well tolerated with minimal toxicity. A minority of patients developed grade 3 or 4 drug-related adverse events including hypertension (18 %), hyperglycemia (13 %), fatigue (11 %), leukopenia (9 %), headache (9 %), proteinuria (7 %), and hypokalemia (7 %). The ORR of 41 % exceeds that reported to date for monotherapy with any targeted agent in pNET and provides compelling evidence to pursue this combination further [41].
5.3.2 Everolimus
Everolimus, a second-generation mTOR inhibitor, was recently approved for use in pNETs after demonstrating significant improvements in outcomes [25, 42]. Everolimus (40-O-(2 hydroxyethyl) derivative of rapamycin, RAD001, Afinitor®, Novartis) is an oral mTOR inhibitor that selectively inhibits mTORC1 and is absorbed rapidly, achieving peak concentration after 1.8 h and reaching steady state after 7 days [16]. It binds to FKBP-12 in a similar mechanism as temsirolimus, by forming a complex that induces the inhibition of mTOR kinase activity. It reduces the activity of mTOR’s downstream proteins by blocking phosphorylation of 4E-BP1 and inactivating S6K1. It also inhibits expression of HIF-1α and decreases expression of VEGF. Everolimus has been shown to reduce cell proliferation, angiogenesis, and glucose uptake from several in vivo and in vitro studies. In addition, it has demonstrated a potent dose-dependent inhibition of cell growth comprising G0/G1 phase arrest as well as induction of apoptosis in human pancreatic BON cells, a human pancreatic NET cell line exhibiting constitutively activation of PI3K/Akt/mTOR pathway [43]. Everolimus treatment also significantly inhibited cell proliferation in the rat insulinoma NET cell line (INS1) and decreased phosphorylation of all downstream targets of Akt, TSC2, and mTOR [44].
In a phase I study evaluating patients with advanced solid tumors including NETs, everolimus induced a dose and schedule-dependent inhibition of the PI3K/Akt/mTOR pathway. At 10 mg/day and ≥50 mg/week, there was an almost complete inhibition of phosphorylated ribosomal protein S6 (p < 0.001), expression of eIF4G (p < 0.001), and reduction of phosphorylated 4E-BP1 (p = 0.058). Furthermore, there was an overall increase in Akt phosphorylation (p = 0.006) and in cellular proliferation (p = 0.014). A total of four out of the 55 patients reached a clinical benefit (a partial response was observed in one patient, and three had stable disease). The dose-limiting toxicities consisted of grade 3 stomatitis, neutropenia, and hyperglycemia which were seen in five patients [45].
A phase II trial evaluated the activity of everolimus in combination with octreotide. Thirty patients with carcinoid and 30 patients with pNETs were treated with everolimus at 5 or 10 mg/day in combination with octreotide LAR 30 mg every 4 weeks. The intent-to-treat response rate was 20 %. The analysis showed that 13 (22 %) patients achieved partial responses, 42 (70 %) patients had stable disease, and 5 (8 %) patients progressed. The median PFS was 60 weeks. Median overall survival had not been reached; however, 1-, 2-, and 3-year survival rates were 83 %, 81 %, and 78 %, respectively. At study entry, among 37 patients with high chromogranin A levels, 26 patients (70 %) attained normalization or a reduction of more than 50 %. In pre- and posttreatment tumor biopsies, mean tumor Ki-67 expression decreased significantly from 6.7 to 2.1 % (p = 0.04). Overall, compared to patients that received the 5 mg dose, patients that received the 10 mg dose obtained a higher response rate (30 vs. 13 %) and had a prolonged median PFS (72 vs. 50 weeks). The most common toxicity was mild aphthous oral ulceration. Significant toxicities were uncommon and only 11 % of patients developed grade 3/4 hypophosphatemia, fatigue, and diarrhea [46].
Three RADIANT (RAD001 in advanced neuroendocrine tumors) trials were then designed to study efficacy of everolimus in NETs of different origins. These confirmed the value of everolimus in patients with advanced NETs. RADIANT-1 is a second open-label phase II trial in 160 patients with progressive chemotherapy-refractory metastatic pNET. Patients were stratified according to octreotide therapy with the primary endpoint assessing response rates in patients in stratum 1. Stratum 1 comprised of 115 patients treated with everolimus 10 mg daily alone and stratum 2 comprised of 45 patients treated with everolimus 10 mg daily plus octreotide LAR ≤30 mg every 28 days. In stratum 1, 11 patients (9.6 %) showed a partial response, 78 patients (67.8 %) had stable disease, and 16 patients (13.9 %) progressed, resulting in a clinical benefit of 77 %. The mean PFS was 9.7 months and overall survival was 24.9 months. In stratum 2, two patients (4.4 %) achieved partial response, 36 patients (80 %) had stable disease, and no patients with progressive disease, resulting in a clinical benefit of 84 %. The mean PFS was 16.7 months and overall survival was not reached after a follow-up period of over 16 months. This study supports the safety and antitumor activity of everolimus alone or in combination with octreotide in patients with progressive pNETs after failure of prior systemic chemotherapy [47].
Following these encouraging results, two pivotal phase III randomized trials were developed. RADIANT-2 evaluated the combination of everolimus plus octreotide LAR compared to octreotide LAR alone in 429 patients with low- to intermediate-grade advanced NET. Although the study failed to reach its primary endpoint, it demonstrated that everolimus plus octreotide LAR significantly improved PFS by 5.1 months (hazard ratio = 0.77, 95 % CI 0.59–1.00, p = 0.026); the mean PFS was 16.4 months in the everolimus plus octreotide LAR group and 11.3 months in the placebo plus octreotide LAR group. After adjusting for differences in baseline characteristics, everolimus plus octreotide LAR also significantly reduced the risk of disease progression by 40 % (hazard ratio = 0.60, 95 % CI 0.44–0.84, p = 0.0014) when compared to octreotide LAR alone [48].
A subgroup analysis of RADIANT-2 trial has shown that early combination therapy with octreotide might be associated with a better outcome compared to patients on octreotide with everolimus added on later (25.2 vs. 13.6 months). COOPERATE-2 study is an ongoing prospective randomized open-label phase II trial in pNET that aims to address the superiority of combination therapy and evaluates the treatment effect of everolimus with a novel somatostatin analog, pasireotide LAR, in comparison to everolimus monotherapy on PFS in patients with advanced progressive pNET (NCT01374451).
RADIANT-3 is the largest phase III pNET trial to date. This was a landmark double-blinded and placebo-controlled study that evaluated 410 patients with advanced, low-grade, or intermediate-grade pNET who received everolimus 10 mg/day (n = 207) or placebo (n = 203). Everolimus more than doubled progression-free survival (11 months vs. 4.6 months (p < 0.0001)) and was associated with a 65 % reduction in progression or death. Although the objective response rate to everolimus was low at 5 % (2 % in the placebo arm), there was a benefit seen in patients with prolonged stable disease in the everolimus arm (73 % vs. 51 % for everolimus and placebo, respectively). The overall survival was not significantly different between the two groups as more than 70 % of patients randomly assigned to placebo crossed over to the treatment arm after disease progression [1, 42]. There was a twofold increase in adverse events; the most common side effects were hematological, diarrhea, stomatitis, or hyperglycemia, ranging from 3 to 7 %. These side effects were manageable with dose reduction, drug interruption, or both [42]. In subgroup analyses, these benefits extended to patients regardless of ethnicity of history of previous therapies [49].
The approval of everolimus has changed the landscape for patients with advanced well-differentiated pNETs. Although the timing of everolimus in the treatment of advanced pNETs is not yet established, everolimus was equally effective in patients regardless of treatment history. The European Neuroendocrine Tumor Society (ENETS) 2012 determined that everolimus represents a novel therapeutic option in patients with unresectable pNETs after progression following chemotherapy, and in selected cases, everolimus should be considered as first-line therapy [50]. Similarly, the National Comprehensive Cancer Network (NCCN) recommended the use of everolimus as a possible first-line treatment for advanced unresectable well-differentiated pNETs (the NCCN clinical practice guidelines in oncology for neuroendocrine tumors version 1.2012. 2012. www.nccn.org).
Although there is convincing data for everolimus in pNETs, its efficacy in other NET subtypes, such as bronchopulmonary or colonic NET, has not been determined. Preclinical studies suggest that susceptibility to everolimus may depend on site of origin for NETs [51]. The RADIANT-4 trial will investigate the benefit of everolimus versus placebo in patients with advanced nonfunctional neuroendocrine tumor of gastrointestinal or lung origin (NCT01524783).
5.4 Resistance Mechanisms of PI3K/Akt/mTOR Pathway Inhibitors
Current mTOR inhibitors have therapeutic limitations as patients initially respond but will eventually progress despite continuous therapy. Primary and acquired resistance appears to limit the efficacy of targeting the PI3K/Akt/mTOR pathway. Escape mechanisms, abrogation of negative feedback loops, and mutations in targeted pathways can all lead to resistance (Table 5.2).
Table 5.2
mTOR inhibitors and neuroendocrine tumors
Therapy year reported (reference) | NET subtype (N = patients) | Response rate (%) | Progression-free survival (months) |
|---|---|---|---|
Temsirolimus 2006 [30] | NET (21) pNET (15) | 5 7 | 6 11 |
Temsirolimus and avastin 2013 [41] | pNET (55) | 41 | 12 |
Everolimus and octreotide LAR 2008 [46] | NET (30) pNET (30) | 17 27 | 15 12 |
Everolimus Everolimus + octreotide LAR 2010 [47] | pNET (115) pNET (45) | 10 4 | 10 17 |
Octreotide LAR Octreotide LAR + everolimus 2011 [48] | NET (213) NET (216) | 2 2 | 11 16 |
Everolimus Placebo 2011 [42] | pNET (207) pNET (203) | 5 2 | 11 5 |
Everolimus + Avastin 2010 [52] | NET (34) | 26 | 14 |
In NET cell lines, mTOR1 inhibitors produce escape mechanisms in both Erk and Akt pathways [51]. For example, rapamycin activity was associated with increased levels of phospho-Akt and phospho-ERK. Akt and ERK are then able to act in concert with RAS and PI3K thereby activating these pathway [53].
Primary resistance mechanisms, such as preexisting mutations in the targeted pathways, may render many targeted therapies ineffective. In tumors harboring K-Ras or B-Raf mutations, resistance is due to activation of alternative pathways, such as the Erk pathway [20].
There is concern for the use of single-agent everolimus in the treatment of NETs due to the presence of feedback loops and crosstalk that exist within and between PI3K/Akt/mTOR and other signaling pathways. Recent data suggest that the loss of negative feedback loops from inhibition of mTORC1 leads to compensatory activation of PI3K and Akt, which drives resistance via upregulation of mTORC2 [54]. Two well-characterized mTORC1 substrates are eukaryotic translation initiation factor 4E-binding protein-1 and ribosomal S6 kinase-1 (S6K1), both regulating transcription and translation initiation of critical growth genes. However, S6K1 is part of a negative feedback loop on PI3K/Akt signaling via suppression of the insulin receptor substrate-1 (IRS1), which links IGF-1 to the PI3K pathway. mTORC2 is less defined than mTORC1, but is known to mediate Akt phosphorylation on serine-473, which is required for full Akt activity in the PIK3/Akt/mTOR signaling cascade. Normally, activation of S6K through mTORC1 phosphorylation results in phosphorylation of rictor, which prevents mTORC2 activation [55, 56]. If mTORC1/S6K is inhibited, the negative feedback is lost leading to increased mTORC2-mediated phosphorylation and activation of Akt [57]. Thus, inhibition of mTORC1 by everolimus may lead to paradoxical upregulation of Akt. This concern has been confirmed in tumor biopsies from patients treated with mTOR inhibitors [58].
Another potential mechanism of resistance is the loss of a negative feedback loop that normally prevents upstream overstimulation of insulin receptor substrate 1 (IRS1) [53]. mTORC1 activation of S6K causes uncoupling of IGF-1 from the PI3K/Akt pathway. Normally, IGF-1 binds IGFR which in turn phosphorylates substrates IRS1-2 which then suppresses PI3K. mTORC1/S6K inhibition results in the loss of this feedback loop and leads to the upregulation of IRS1 protein and activation of the PI3K/Akt cascade [53, 54, 59]. Accordingly, several approaches to downregulate IGF with somatostatin analogs such as octreotide and pasireotide, or inhibit IGF-1R signaling with a monoclonal antibody, such as cixutumumab (IC-A12) are being developed in combination with everolimus. There is an ongoing phase I study with the combination of cixutumumab, everolimus, and octreotide LAR in patients with advanced NETs (NCT01204476).
5.4.1 Novel Approaches
The PI3K/Akt/mTOR pathway is complex and perturbations can occur at multiple sites. Therefore, there are several potential targets and combinations of therapies compelling for further investigation. The use of PI3K inhibitors, Akt inhibitors, or mTORC1 and mTORC2 inhibitors as single agents or in combination can avert PI3K/Akt/mTOR pathway activation and reactivation [55]. A host of novel inhibitors that target key nodes with the PI3K/Akt/mTOR pathway have shown encouraging results in preclinical studies and are currently in early phase clinical trials.
Inhibitors of Akt either compete with ATP at the active site or bind distally to the catalytic site, inducing a conformational change that prevents ligand binding. Akt inhibition may be expected to abrogate negative feedback loops perpetuated by mTORC2 following mTORC1 inhibition [58]. Agents that inhibit both mTOR complexes may also overcome this problem. Therefore, inhibitors of both mTORC1 and mTORC2 and Akt inhibitors are attractive drug candidates.
Potential PI3K/Akt/mTOR pathway target upstreams of mTOR are the PI3K proteins themselves. Three classes (I–III) of PI3K have been characterized that vary in structure and substrate preference. The class I enzymes are activated directly by cell-surface receptors, and it is the catalytic domain of the class IA PI3K p110 subunits that are the most widely implicated in cancer [56]. Pan-PI3K inhibitors target all four class I p110 isoforms; however, PI3K inhibitors specific for individual class I p110 isoforms may allow for anticancer activity with an improved safety profile. The majority of therapeutic interventions or drugs under investigation are pan-p110 inhibitors, although a number of PI3K-targeted agents with isoform specificity have now been reported [55, 57]. It is of potential clinical significance that dual inhibition of PI3K and mTORC1/2 may be mediated through the shared structural homology between the catalytic domains of the PI3K p110 subunits and mTORC1/2 [60]. Agents that target both PI3K and mTOR will likely lead to improved inhibition of this pathway.
5.4.2 mTORC1 and mTORC2 Inhibitors
CC-223 is currently an experimental dual mTOR kinase inhibitor, inhibiting both TORC1 and TORC2 complexes. In a recent phase I trial, 101 solid tumor subjects were treated with CC-223 dosed at 45 mg once daily in 28 day cycles until disease progression. From the non-pancreatic NET cohort (n = 23), patients with progression within 12 months and receiving ongoing treatment with somatostatin analogs were included in the study. Biomarkers confirmed inhibition of TORC1 and TORC2 by p4EBP1 and pAKT, respectively. In 7 out of 13 subjects (54 %), PET imaging demonstrated a response at day 15 (>25 % change in SUV). All evaluable patients were stable on CC-223, with treatment ongoing up to nine cycles (median 6; range 4–9) (n = 10). Although not prospectively collected, there were six subjects with refractory carcinoid syndrome that reported complete resolution of flushing [5] and improvement in diarrhea [1]. Symptom improvement generally occurred within the first week of dosing and persisted despite dose reduction in five subjects [61].
The most common related adverse events of all grades were stomatitis, diarrhea, fatigue, anorexia, nausea, and rash. In addition, related serious adverse events included one case of transient dehydration/renal insufficiency. CC-223 dose reduction to 30 or 15 mg was required in 57 % of subjects, usually during cycle 1 or 2, but thereafter treatment was well tolerated [61].
These results are from an ongoing phase I/II study to assess the safety and efficacy of CC-223 in patients with advanced tumors (other than pNETs) unresponsive to standard therapies and to determine the appropriate dose and tumor type for later-stage clinical trials (NCT01177397).
5.4.3 HSP 90 Inhibitors
There have been numerous preclinical data supporting the role of novel PI3K/Akt/mTOR pathway inhibitors in NETs, either through direct inhibition of specific pathway proteins or through indirect inhibition of molecular chaperones. The molecular chaperone heat-shock protein 90 (HSP90) is an emerging target for anticancer therapy as it is overexpressed in a number of tumors. The HSP90 inhibitor, IPI-504, has been studied in pNET cells. The potential activity of IPI-504 has shown to inhibit the growth of human insulinoma and pancreatic carcinoid cells by almost 70 %. IPI-504 also has antiproliferative effects by downregulating IGF-1 and several downstream factors of the PI3K/Akt/mTOR. Combination of IPI-504 with mTOR or Akt inhibitors also resulted in increasing antiproliferative effects [62]. This is a promising agent for the treatment of NETs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree