Patient population
N
Design
Treatment arms
Stratification factors
Endpoints
Results
BOLERO-1
HER2+ ABC. First line
719
Randomization 2:1
Everolimus or placebo 10 mg/day + paclitaxel 80 mg/m2 days 1, 8, 15 + trastuzumab 2 mg/kg weekly: 28-day cycle
Prior adjuvant or neoadjuvant trastuzumab
Visceral metastases
Primary: PFS
Secondary: ORR, OS, safety, PK, biomarkers
Not yet reported
BOLERO-2
Postmenopausal, HR+, HER2-, ABC, refractory to letrozole or anastrozole
724
Randomization 2:1
Everolimus or placebo 10 mg/day + exemestane 25 mg/day
Sensitivity to prior hormonal therapy
Presence of visceral disease
Primary: PFS
Secondary: OS, ORR, CBR, QoL, bone markers
PFS = 11 months versus 4.1 months in favor of everolimusa
BOLERO-3
HER2+ ABC. Prior taxane therapy and resistance to trastuzumab
569
Randomization 1:1
Everolimus or placebo 5 mg/day + vinorelbine 25 mg/m2 days 1, 8, 15 + trastuzumab 2 mg/kg days 1, 8, 15: 21-day cycle
Prior lapatinib
Number of prior chemotherapy regimens for advanced disease (1 versus 2–3)
Primary: PFS
Secondary: OS, ORR, safety, PROs, lab measures
PFS: 7 months versus 5.78 months in favor of everolimusa
Ongoing trials are testing everolimus in combination with other endocrine agents in metastatic breast cancer, beyond progression on previous everolimus treatment. The approved combination with exemestane is also tested versus chemotherapy (Table 4.2).
Table 4.2
Ongoing clinical trials with everolimus in ER+ breast cancer
Phase | Setting | Trial population | Treatment regimen | Objective | Trial identification |
|---|---|---|---|---|---|
2 | Metastatic | First line | Part 1: letrozole + everolimus 10 mg/day Part 2: exemestane + everolimus 10 mg/day | Primary: PFS 2nd line Secondary: ORR 1st and second line PFS 2nd line | NCT01698918 |
2 | Progression on NSAI | Everolimus 10 mg/day vs Everolimus 10 mg/day + exemestane vs capecitabine 2500 mg/m2 days 1–14 q21 days | Primary: PFS Secondary: OS QoL | NCT01783444 | |
2 | Progression on AI | Fulvestrant + everolimus 10 mg/day | Primary: TTP Secondary: OS | NCT00570921 | |
2 | Hormone refractory, HER2 IHC 1 or 2+ | Last hormonal treatment + trastuzumab vs Last hormonal treatment + trastuzumab + everolimus 10 mg/day | Primary: ORR | NCT00912340 | |
2 | Progression on AI, obese | Exemestane + everolimus 10 mg/day + metformin | Primary: PFS | NCT01627067 | |
2 | Progression on tamoxifen or anastrozole or exemestane | Letrozole + everolimus 10 mg/day | Primary: ORR | NCT01231659 | |
2 | HER-2– or +, progressing on tamoxifen or AI | Letrozole + lapatinib then addition of everolimus 10 mg/day on progression | Primary: CBR after addition of everolimus | NCT01499160 | |
2 | Metastatic | Bone metastases | Everolimus 10 mg/day | Primary: TTP | NCT00466102 |
2 | AI resistant | Fulvestrant + placebo/everolimus 10 mg/day | Primary: PFS | NCT01797120 | |
2 | Progression after exemestane + everolimus | Endocrine treatment + placebo/everolimus 10 mg/day | Primary: PFS | NCT01773460 | |
3 | Adjuvant | At least 4+ LNs after surgery or at least 1+ LN if neoadjuvant chemotherapy | Everolimus 10 mg/day or placebo add-on to endocrine treatment after 3 years of start to complete 5 years | Primary: DFS | NCT01805271 |
3 | High-risk early breast cancer | Everolimus 10 mg/day or placebo for 1 year add-on to adjuvant endocrine treatment | NCT01674140 |
4.1.4.2 The Neoadjuvant Setting
A randomized phase 2 trial was performed and reported in the neoadjuvant setting. Two hundred and seventy postmenopausal patients with M0 ER+ breast cancer were randomized to letrozole + placebo versus letrozole + everolimus. Tumor core biopsies were performed baseline and at day 15 in order to evaluate PI3K mutations and pharmacodynamic changes. The response rate in the everolimus arm superior and the difference was statistically significant: 68 % vs 59 % by clinical palpation and 58 % vs 47 % by ultrasound. There were few pathological complete responses: two in the everolimus arm and one in the placebo arm. Pharmacodynamic analyses showed a marked reduction in phosphorylated ribosomal protein S6 in the everolimus arm. There were more adverse events recorded in the everolimus arm [34].
4.1.4.3 The Adjuvant Setting
Clinical trials are enrolling patients to extend everolimus indications to the adjuvant setting of ER+ breast cancer (Table 4.2).
4.1.5 Clinical Data with Temsirolimus
Temsirolimus (Torisel®, Pfizer) was tested as monotherapy in two phase 2 trials enrolling patients with pretreated metastatic breast cancer regardless of subtype: luminal, HER-2 positive, and triple negative [35, 36]. These trials showed a modest response rate (0–9 %) with a tolerable safety profile. Adverse events were as expected with mTOR inhibitors: fatigue, rash, mucositis, and hyperglycemia. Early data in combination with letrozole in the treatment of ER+ metastatic breast cancer proved encouraging: a small randomized, open-label, three-arm phase 2 study (n = 92) of letrozole + oral temsirolimus 10 mg daily or 30 mg intermittently showed similar toxicity profiles in both temsirolimus arms (42 % and 57 % of patients with any mucositis, respectively), with a doubling of the PFS in the intermittent arm compared with letrozole alone [37]. The HORIZON phase 3 trial was therefore initiated to test the efficacy and safety of first-line oral letrozole 2.5 mg daily in combination with temsirolimus 30 mg daily (5 days every 2 weeks) versus letrozole and placebo in 1112 patients with AI-naive, hormone receptor-positive advanced disease [38]. However, a recommendation by an independent data monitoring committee led to the early termination of this trial for futility at the second preplanned interim analysis. There was no overall improvement in the primary endpoint PFS (median, 9 months; hazard ratio [HR], 0.90; 95 % CI, 0.76–1.07; P = 0.25) nor in the 40 % patient subset with prior adjuvant endocrine therapy. These findings were contradictory with the BOLERO-2 data, albeit that the trial was conducted with different agents. A difference in drug metabolism (CYP3A genotypes) between the two trial populations, a different percentage of patients with the luminal subtypes (luminal A versus luminal B), and differential drug effectiveness are attempted explanations for these findings. However, the most likely explanation is endocrine sensitivity. One could argue that the mTOR inhibitor benefit may be restricted to those with acquired AI resistance: while the BOLERO-2 trial enrolled 84 % of patients with initial endocrine sensitivity but progressing afterward on an AI, the HORIZON trial patient population was largely AI naïve [39].
4.1.6 mTOR Inhibitors in Combination with Other Signal Transduction Inhibitors
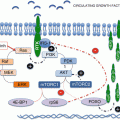
The insulin-like growth factor receptor 1 (IGF-1R) pathway is a major contributor to breast cancer pathogenesis. These receptors are expressed in virtually all breast cancer cell lines, and they are believed to enhance growth and inhibit apoptosis. IGF-1R expression is 14-fold higher in malignant breast tissue, and IGF-1R autophosphorylation and kinase activity are 2–4-fold higher than in normal breast tissue. This results in a 40-fold elevation in IGF-1R tyrosine kinase activity, even in the absence of hormonal stimulation [40]. High levels of IGF-1R have been associated with resistance to radiation and breast cancer recurrence [41]. Furthermore, in vitro, IGF-1 restored growth of ER-positive breast cancer cell lines treated with PI3K and ERK1/ERK2 inhibitors [42], explaining in part the low response rate to single-agent mTOR inhibitors. Furthermore, stimulation of the insulin and IGF-1 receptor activates the PI3K-Akt-mTOR pathway. Preclinical models but also human tumor biopsies were used to demonstrate that mTOR inhibition induces insulin receptor substrate-1 expression and abrogates feedback inhibition of the pathway, resulting in Akt activation both in cancer cell lines and in patient tumors treated with everolimus [43]. IGF-I receptor inhibition prevents rapamycin-induced Akt activation and sensitizes tumor cells to inhibition of mTOR, providing a scientific rationale for the combinatorial approach. Trials with different agents targeting mTOR and IGF-R1 have been designed for the treatment of ER+ breast cancer, some of which do not include an endocrine agent in the trial design.
A phase 1 trial with the combination of temsirolimus and cixutumumab (ImClone, Inc.), a fully humanized monoclonal antibody that binds to the IGF-1R with high affinity (K d = 0.04 nM) and blocks ligand binding to the receptor, enrolled 26 patients with metastatic breast cancer, 86 % of whom had ER+ disease. No objective responses were recorded in this heavily pretreated population, but stable disease for more than 4 months was observed in four patients. DLTs included mucositis, neutropenia, and thrombocytopenia, while other adverse events included grade 1–2 fatigue, anemia, and hyperglycemia. Most toxicities were manageable, and there was no DLT nor severe toxicity at the maximum tolerated dose (MTD) of cixutumumab 4 mg/kg and temsirolimus 15 mg weekly [44]. An expansion two-stage Simon phase 2 clinical trial design is now underway to assess the antitumor activity of the combination at the recommended phase 2 dose in patients with metastatic breast cancer [45].
Ridaforolimus (AP23573/MK-8669, formerly deforolimus) is an orally available non-prodrug analogue of rapamycin and a potent and selective mTOR inhibitor. There were no recorded objective responses in metastatic breast cancer patients treated intravenously in two phase 1 trials with single-agent ridaforolimus [46, 47] and two phase 1 trials with orally administered single-agent ridaforolimus [48, 49]. Dalotuzumab is a recombinant monoclonal antibody directed against IGF-1R. In preclinical studies, the combination of ridaforolimus and dalotuzumab showed additive or synergistic antitumor activity in most tested cell lines and tumor xenograft models. The presence of IGF-1R and the activation of the IGF-1R pathway were necessary for combination benefit [50]. The phase 1 trial of the ridaforolimus and dalotuzumab combination was reported and showed promising activity in ER+/high proliferation breast cancer. Two confirmed partial responses were recorded and two other breast cancer patients achieved metabolic partial response on FDG-PET scan. Of the 23 enrolled breast cancer patients, 5 derived benefit, all of which were ER+ with 4 having high Ki67. DLTs were stomatitis and fatigue and an expansion cohort below the MTD was tested at ridaforolimus 30 mg/day from day 1 to 5 every week plus dalotuzumab 10 mg/kg/week [51]. A randomized phase 2 study with the combination of ridaforolimus and exemestane, compared to the triplet combination of ridaforolimus, dalotuzumab, and exemestane, has recently finished recruitment in patients with ER+ high proliferation breast cancer progressing on aromatase inhibitor therapy. This clinical design is supported by the hypothesis that ER+ high proliferation breast cancer is characterized by features indicative of high PI3K pathway activity, relatively low utilization of ER leading to endocrine resistance, high expression of IGF-1R family members, and low RAS pathway activity rendering this tumor type susceptible to dual inhibition of IGF-1R and mTOR pathways with the ridaforolimus–dalotuzumab combination [50, 52].
BI 836845 is a fully human antibody, currently in advanced phase 1 development, which potently neutralizes both IGF-1 and IGF-2. It was able to improve the efficacy of rapamycin by inhibiting upstream signaling in preclinical models [53]. A phase 1b/2 trial to determine the MTD and recommended phase 2 dose, and to evaluate the safety and antitumor activity, of BI 836845 and everolimus in combination with exemestane in women with HR+/HER-2− advanced breast cancer will soon start enrollment (NCT02123823).
Phase 2 trials are yet to be reported, but results so far have not met yet the preclinical expectations.
4.2 HER-2-Positive Breast Cancer
4.2.1 Background
HER-2 gene amplification and/or protein overexpression has been described in 10–34 % of breast cancers and is both a prognostic and a predictive factor. HER-2-positive breast tumors are associated with pathologic and clinical characteristics such as high cell proliferation, cell motility, tumor invasiveness, high probability of progressive regional and distant metastases, accelerated angiogenesis, and reduced apoptosis. When compared to endocrine-responsive disease, they have a higher grade and are diagnosed more often with lymph node metastases. In 107 studies considering 39,730 patients, these cancers were a negative prognostic factor independently of other prognostic variables [54]. This was, however, before the advent of trastuzumab and subsequent anti-HER-2 therapies.
Since the first phase 2 clinical experience in 1996 [55], trastuzumab, an anti-HER2 recombinant humanized monoclonal antibody, has become, in combination, the mainstay of treatment of HER-2-positive breast cancer in the metastatic [56] and adjuvant settings [57–60]. However, despite the dramatic improvement in prognosis of patients treated with adjuvant trastuzumab, as many as 7–20 % of patients still present with a breast cancer relapse. Other anti-HER-2-targeted agents have been approved by the regulatory agencies for the treatment of metastatic HER-2-positive breast cancer: lapatinib, a tyrosine kinase inhibitor of HER-2 [61]; pertuzumab, an anti-HER2 humanized monoclonal antibody that inhibits receptor dimerization [62]; and T-DM1, an antibody–drug conjugate incorporating the HER2-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule inhibitory agent DM1 [63]. Pertuzumab has also received FDA approval in the neoadjuvant setting for locally advanced, inflammatory, or early stage breast cancer in combination with trastuzumab and chemotherapy. Other agents are in clinical testing such as neratinib [64] and afatinib [65]. Some tumors are still able to evade inhibition prompting the search for combinatorial approaches to reverse resistance.
4.2.2 Resistance Cross-Talk
Different mechanisms of resistance to anti-HER-2 therapies are hypothesized in the treatment of HER-2 positive breast cancer: increased expression of p95HER-2, a truncated HER-2 receptor with constitutive activity; increased HER-2 expression due to HSP90 (heat shock protein 90) overexpression, disrupted antibody–receptor interaction, and failure to elicit an immune response; increased signaling through other growth factor receptors (IGF-1R, VEGFR, MET); alterations in downstream molecules (PTEN downregulation, PIK3CA mutations, increased Akt signaling); and increased cell survival due to telomerase expression [66].
A large amount of preclinical and clinical data demonstrates the role of the PI3K-AKT-mTOR pathway in the resistance to anti-HER-2-targeted agents. This pathway is involved in both the de novo and acquired resistance mechanisms. In vitro, loss of PTEN as well as PIK3CA mutations were associated with resistance to trastuzumab [67] and lapatinib [68, 69]. Patients with PTEN loss or PIK3CA mutations treated with trastuzumab had a shorter PFS [70]; and in another study, patients with PTEN loss had a lower response rate [71]. Acquired resistance was seen in three in vitro trials: Akt-negative feedback loop that perpetuated HER-2 phosphorylation leads to a decreased response to trastuzumab [72], while modifications in the pathway and the balance between phosphorylated and non-phosphorylated PTEN after exposure to HER-2-targeted therapy lead to acquired resistance to trastuzumab [67, 73].
Robust preclinical experiments support the restoration of trastuzumab sensitivity with the combination of mTOR inhibitors and trastuzumab. Everolimus restored the sensitivity of trastuzumab in an in vitro model of breast cancer with PTEN loss, and the combination inhibited tumor growth in a mouse xenograft model more than either agent alone [74]. The same experiment yielded the same results in vivo and in vitro with the treatment of trastuzumab-resistant models with the combination of trastuzumab and rapamycin or everolimus [75].
4.2.3 mTOR Inhibitors and Anti-HER-2 Combinations
Everolimus and trastuzumab is the combination that is most advanced so far in clinical development. A pooled analysis combined data from two phase 1b/2 clinical trials that enrolled women with HER-2+ metastatic breast cancer after progression on trastuzumab-based therapy. Forty-seven patients were treated with trastuzumab every three weeks and daily everolimus at two doses, 5 and 10 mg. Seven partial responses (15 %) were recorded, and persistent stable disease was seen in nine patients (19 %) for a clinical benefit rate of 34 % in patients deemed resistant to trastuzumab, 56 % of whom had relapsed within 1 year of completing adjuvant trastuzumab. The combination was found to be tolerable with 9 % of patients presenting with grade 3 stomatitis, 9 % with grade 3 diarrhea, 13 % with grade 3/4 hyperglycemia, and 9 % with grade 3 fatigue. No cardiac toxicity was recorded. Hematological toxicity included grade 3 neutropenia in 9 % of patients and grade 3 thrombocytopenia in 4 %. Dose reductions/delays occurred in 25 patients (53 %). Everolimus 10 mg a day was the recommended dosage [76].
Everolimus combined with weekly paclitaxel and trastuzumab showed very encouraging antitumor activity in a phase 1b trial enrolling 33 patients with HER-2+ metastatic breast cancer, 31 of whom were pretreated with taxanes and 32 were resistant to trastuzumab. Everolimus was tested at three dosages (5 mg/day, 10 mg/day, or 30 mg/week) in combination with paclitaxel 80 mg/m2 on days 1, 8, and 15 every 4 weeks and trastuzumab 2 mg/kg weekly. Overall response rate was 44 %, and the disease was controlled for 6 months or more in 74 % of patients. Overall response rate in 11 patients resistant to both taxanes and trastuzumab was 55 %. There were three recorded DLTs: febrile neutropenia (5 mg/day), stomatitis (10 mg/day), and confusion (30 mg/week). Grade 3–4 neutropenia was observed in 17 patients (52 %), and everolimus 10 mg a day was the recommended dosage for further development [77]. Another phase 1 trial tested the tolerability of everolimus (5 mg/day, 20 mg/week, or 30 mg/week) combined with vinorelbine (25 mg/m2 on days 1 and 8 every 3 weeks) and trastuzumab (2 mg/kg weekly) in 50 women with heavily pretreated HER-2+ metastatic breast cancer progressing after trastuzumab-based treatment. Encouraging antitumor activity was also seen in this setting with an overall response rate of 19 %, a disease control rate of 83 %, and a median PFS of 31 weeks. As with paclitaxel, the most common adverse event was grade 3/4 neutropenia, and other DLTs included febrile neutropenia, grade 3 stomatitis with concomitant fatigue, grade 2 stomatitis, grade 3 anorexia, and grade 2 acneiform dermatitis. Everolimus 5 mg/day and 30 mg/week were chosen as the recommended dosages [78].
On the basis of these results, phase 3 trials with these combinations were designed. The BOLERO-1 trial is a phase 3 trial randomizing patients with metastatic HER-2-positive breast cancer in the first-line setting to paclitaxel + trastuzumab + everolimus (10 mg daily)/placebo. It has been completed and results are awaited [79]. The BOLERO-3 trial enrolled HER-2+ metastatic breast cancer patients pretreated with taxane and trastuzumab resistant. A total of 569 patients were randomly assigned to receive either 5 mg everolimus daily with 25 mg/m2 vinorelbine weekly and 2 mg/kg trastuzumab weekly (284 patients) or placebo plus the same vinorelbine and trastuzumab regimen (285 patients). A total of 27 % of patients in each group had received prior lapatinib. The everolimus group had a median PFS of 7.00 months compared with 5.78 months in the placebo group (HR 0.78, 95 % CI [0.65, 0.95]; p = 0.0067). The overall response rate (complete or partial response) was 40.8 % in the everolimus group and 37.2 % in the placebo group (p = 0.2108), and the clinical benefit rate (objective response or stable disease at 24 weeks or more) was also not significantly different between the groups (59.2 % everolimus vs. 53.3 % placebo; p = 0.0945). Quality-of-life measures also showed no differences between the treatment arms [80]. Class effect adverse events associated with mTOR inhibitors (e.g., stomatitis, rash, noninfectious pneumonitis, and hyperglycemia) occurred more frequently in the everolimus arm, and most were grade 1/2. The incidences of serious adverse events suspected to be treatment-related were 26.4 % in the everolimus arm and 6.4 % in the placebo arm. Grade 3 class effect adverse events in the everolimus arm each occurred in less than 15 % of patients: (stomatitis, 13 %; hyperglycemia, 4 %) [81]. The most common grade 3–4 adverse events were neutropenia (204 [73 %] of 280 patients in the everolimus group vs 175 [62 %] of 282 patients in the placebo group), leucopenia (106 [38 %] vs 82 [29 %]), anemia (53 [19 %] vs 17 [6 %]), febrile neutropenia (44 [16 %] vs 10 [4 %]), stomatitis (37 [13 %] vs 4 [1 %]), and fatigue (34 [12 %] vs 11 [4 %]). Serious adverse events were reported in 117 (42 %) patients in the everolimus group and 55 (20 %) in the placebo group [82]. Although the primary endpoint of the trial was met—with a statistically improved PFS for the everolimus arm—the magnitude of improvement is disappointing: 6 weeks only. It should be noted that the trial enrolled women with hormone receptor-negative as well as hormone receptor-positive disease: one may wonder whether, for the latter, the ER pathway did not provide an “escape” mechanism. Even though this trial confirmed the proof of concept established in earlier trials, the toxicity profile and the “limited” efficacy could be obstacles for regulatory approvals in comparison with the more favorable safety and efficacy data of agents such as pertuzumab and T-DM1.
Evidence also emerged from the neoadjuvant setting with the RADHER trial in which 82 patients with HER-2+ early breast cancer were randomized to a short course (6 weeks) of preoperative treatment with trastuzumab or the combination of trastuzumab and everolimus. The combination improved the clinical response rate (35 % versus 22.5 %) [83].
Everolimus and other mTOR inhibitors have been tested in phase 1 and 2 trials in combination with trastuzumab or with other anti-HER-2 agents (Table 4.3) [84–89].
Table 4.3
Ongoing and completed trials with the combination of mTOR inhibitors and anti-HER-2 agents
Reference | Phase | Setting | Trial population | mTOR inhibitor | Anti-HER-2 agent | Chemotherapy | Endocrine agent |
|---|---|---|---|---|---|---|---|
81 | 1b/2 | Metastatic | HER-2+, trastuzumab refractory | Temsirolimus | Neratinib | None | None |
82 | 2 | Metastatic | HER-2+, trastuzumab refractory | Ridaforolimus | Trastuzumab | None | None |
83 | 2 | Metastatic | HER-2+, CNS metastases allowed | Everolimus | Lapatinib | None | None |
84 | 2 | Metastatic | HER-2+ with brain metastases | Everolimus | Trastuzumab | Vinorelbine | None |
85 | 1b/2 | Metastatic | HER-2+ with brain metastases | Everolimus | Lapatinib | Capecitabine | None |
86 | 2 | Metastatic | ER+, HER-2 + or − after progression on lapatinib and letrozole | Everolimus | Lapatinib | None | Letrozole |
4.3 ER-Negative and HER-2 Negative Breast Cancer: “Triple Negative Breast Cancer”
4.3.1 Background
Triple negative breast cancer (TNBC) refers to a subgroup of breast tumors that do not express ER, PR, and HER-2. Almost 15 % of breast cancers are classified as TNBC according to this definition, but recent experiments using gene expression profiling have led to further subtyping into six new groups: basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stemlike, and luminal androgen receptor [90]. While the treatment of TNBC has been challenging because of the heterogeneity of the disease and the absence of well-defined molecular targets, this development is considered a landmark on the path to discovering new drug targets. Indeed, despite some sensitivity to systemic chemotherapy, mainly taxane and anthracycline-based regimens, patients with TNBC are generally at a greater risk of early systemic relapse and poorer survival than patients with ER+ or HER-2+ breast cancer [91, 92]. These cancers are characterized by a low correlation between the size of the primary and the metastatic potential, rapid growth, and frequent occurrence in young women, thus evading screening detection and higher likelihood of metastasizing to viscera (mainly the lung and the brain) [93]. New targeted agents are therefore an unmet need against a very lethal and heterogeneous subtype of breast cancer.
4.3.2 Rationale
There are multiple candidate targets and pathways in TNBC [94]:
Hormone receptors: the androgen receptor
DNA repair pathway: PARP1 and Chek1
Host: VEGFA
Cancer stem cells: NOTCH
Tyrosine kinase receptors: FGFR2, EGFR, and IGFR1
Intracellular kinases: PTEN/mTOR
Phosphatase and tensin homologue (PTEN) is a protein that inhibits activation of the AKT/mTOR pathway, and PTEN losses have been observed in up to 30 % of TNBCs [95]. This aberration has been associated with activation of AKT in TNBC samples [96]. Furthermore, the Cancer Genome Atlas Network analyzed 825 primary breast cancers by genomic DNA copy number arrays, DNA methylation, exome sequencing, messenger RNA arrays, microRNA sequencing, and reverse phase protein arrays. In addition to identifying nearly all genes previously implicated in breast cancer, a number of novel significantly mutated genes were identified. The TNBC subtype was found to have the highest mutation rate, albeit that those mutations were more diverse in the other subtypes. TP53 mutations (80 %) were the most common mutation followed by PIK3CA mutations (9 %). The data showed, however, that PI(3)K pathway activity, whether from gene, protein, or high PI(3)K/AKT pathway activities, was highest in basal-like cancers: loss of PTEN and INPP4B and/or amplification of PIK3CA [97]. There is therefore a rationale to develop mTOR inhibition in patients with TNBC that show PTEN loss.
4.3.3 Preclinical Evidence
mTOR inhibitors may also play a role in rational combinations as well as chemotherapy sensitizers. EGFR inhibitors, once regarded as promising agents in the treatment of metastatic TNBC, have failed to impact outcome when administered as single agents [98]. TNBC cell lines and nude mice models were treated with co-inhibition of mTOR and EGFR using rapamycin and lapatinib, respectively. This combination was synergistic in decreasing cell survival and resulted in increased apoptosis in some TNBC cell lines and was associated with the downregulation of rapamycin-induced activation of Akt in vitro [99]. The authors concluded that mTOR inhibitors could improve the efficacy of EGFR-targeting agents in the treatment of some metastatic breast cancers.
4.3.4 Clinical Data
Fifteen patients with metastatic breast cancer, including 15 % with TNBC, were enrolled in a phase 1b trial testing the combination of everolimus and erlotinib [100]. Unfortunately, all but one patient progressed at the first disease evaluation, and future development of this combination was abandoned.
Given that TNBCs are characterized by a deficiency in the DNA repair machinery, DNA alkylating chemotherapy is hypothesized to be particularly effective in this setting. Cisplatin monotherapy achieved a pathologic complete response in 22 % of TNBC patients treated in the neoadjuvant setting [101]. mTOR activation could be one mechanism of resistance to cisplatin, and the addition of everolimus to cisplatin increased its in vitro efficacy fivefold [102]. Fifty-five patients with heavily pretreated metastatic ER− breast cancer (62 % with a median of three prior lines) were treated in a phase 2 trial with weekly cisplatin (25 mg/m2), paclitaxel (80 mg/m2), and daily everolimus (5 mg), given on a 28-day cycle. Sixty-three percent of patients had triple-negative disease, and 81 % patients had visceral disease. Significant antitumor activity was recorded: 11 patients had a partial response and 21 had stable disease. The regimen was tolerable and toxicity was mainly hematological: 24 % grade 3/4 neutropenia and 13 % grade 3 anemia [103]. This combination is currently studied in a randomized neoadjuvant trial with paclitaxel and cisplatin with or without everolimus in stage 2 and 3 TNBC [104]. Another ongoing trial randomizes patients with residual disease after neoadjuvant anthracycline and taxane chemotherapy to everolimus or placebo. However, this trial enrolls breast cancer patients with any subtype [105].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree