Highlights
- •
Intravesical chemotherapy significantly reduces intravesical recurrence risk after NUT at 12 (OR 0.46) and 24 months (OR 0.41).
- •
No statistically significant difference was found between single intra and postoperative intravesical instillations.
- •
The safety profile appears to be favorable with a pooled rate of minor and major complications of 9% and 0.9%, respectively.
Abstract
Introduction
Intravesical recurrence of upper tract urothelial carcinoma after radical nephroureterectomy occurs in 22% to 47%. Intravesical chemotherapy is still underused due to concerns about its efficacy and safety. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of intravesical chemotherapy regimens in reducing the risk of intravesical recurrence following radical nephroureterectomy.
Materials and methods
A literature search was conducted using PubMed/Medline, Embase, and Web of Science databases to identify reports published until March 2024. The PRISMA guidelines were followed to identify eligible studies. The outcomes measured were intravesical recurrence rates and complications in patients treated with different intravesical instillation chemotherapy and timing after radical nephroureterectomy. Sub-analyses were performed on randomized controlled trials and studies involving patients with no history of bladder cancer.
Results
Eighteen studies met our inclusion criteria, and data from 2,483 patients were reviewed. Intravesical chemotherapy significantly reduced the risk of intravesical recurrence at 12 months (OR = 0.46; 95% CI: 0.33–0.65; P < 0.001;) and at 24 months (OR = 0.41, 95% CI: 0.28–0.61; P < 0.001). Notably, no association was found when confronting intra and postoperative instillations (OR = 0.66; 95% CI: 0.34–1.28; P = 0.2), nor single vs. multiple instillation (OR = 1.37; 95% CI: 0.75–2.50; P = 0.3). The pooled rate for minor and major complications was 9% and 0.9%, respectively.
Conclusion
This study demonstrates that intravesical chemotherapy significantly reduces intravesical recurrence rates after radical nephroureterectomy at 12 and 24 months. Additionally, it underscores the favorable safety profile of intravesical chemotherapy, with a low incidence of major complications. The ideal instillation scheme and chemotherapy agent should be further defined.
1
Introduction
Upper tract urothelial carcinoma (UTUC) represents 5% to 10% of all urothelial cancers [ , ]. Radical nephroureterectomy (RNU) with bladder cuff excision is the standard of care for high-risk UTUC [ ]. Despite the surgical expedients, intravesical recurrence after RNU is still a frequent condition that occurs in 22% to 47% of patients [ ].
A recent meta-analysis showed that perioperative instillation of intravesical chemotherapy following RNU reduces intravesical recurrence by 12.7% at 12 months [ ]. Based on the evidence provided by the 2 randomized controlled trials of O’Brian et al. and Ito et al., the American Urological Association and European Association of Urology guidelines recommend the use of a single dose of intravesical chemotherapy after RNU, with the favored regimens being Mitomycin C (MMC) or pirarubicin [ , ]. Despite these strong recommendations, data from the ROBUUST group reported an overall utilization rate of intravesical chemotherapy of only 24% [ ]. The primary reasons for low compliance with the guidelines, as reported in a 2017 survey, were the lack of supporting data (55%), fear of potential side effects (18%), and organizational problems (15%) [ ].
Numerous agents have been used at the time of trans urethral resection of bladder tumor (TURBT) to reduce the risk of NMIBC recurrence but in the context of UTUC there is little data to support 1 intravesical chemotherapeutic over another [ ].
Some clinicians, however, have shifted to using gemcitabine instead of MMC, driven by recent compelling evidence supporting a single dose of intravesical gemcitabine during TURBT for NMIBC to reduce intravesical recurrence rates, alongside concerns about the risk of chemical peritonitis from extravesical extravasation of MMC [ , ]. However, without direct comparisons, the optimal timing of therapy and the selection of the agent remain undefined.
We conducted a systematic review and meta-analysis of the most updated evidence to evaluate the safety and efficacy of the different intravesical chemotherapy regimens in preventing the risk of intravesical recurrence after RNU.
2
Materials and methods
2.1
Search strategy
This systematic review was conducted in line with the updated Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [ ]. The protocol was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO) database (Registration Number CRD42024523231). The PRISMA checklist is provided in eTable 1 , Supplementary Materials. We systematically reviewed the English-language literature using the PubMed/Medline, Web Of Science, and Embase databases to identify reports published from inception to March 2024, which assessed the impact of post-RNU intravesical chemotherapy on intravesical recurrence. A detailed overview of the search strategy is available in eAppendix 1, Supplementary Materials .
2.2
Study selection and inclusion criteria
The primary outcome was post-RNU intravesical recurrence at 12 and 24 months. To address our clinical question, we applied the PICOS framework. We included studies that assessed patients who underwent RNU for UTUC (Patients) and received post-RNU intravesical instillation (Intervention). These patients were compared with those who either did not receive intravesical instillations or were treated with different therapeutic regimens in terms of the agent used, timing relative to the RNU, the number of instillations, or the dosage (Comparator) to assess intravesical recurrence (Outcome) in both prospective and retrospective studies (Study design). The secondary outcomes were to evaluate the overall, minor and major complication rates based on the Clavien-Dindo classification. Initial screening was performed independently by 2 investigators (S.M. and A.P.) based on the titles and abstracts of the articles to identify ineligible reports. Reviews, meta-analyses, editorials, commentaries, authors’ replies, meeting abstracts of unpublished studies, and case reports were excluded, but the reference section was checked to avoid omitting relevant articles. Potentially relevant studies were subjected to a full-text review, and the relevance of the reports was confirmed after the data extraction process. Duplicated studies from the same author’s group were excluded, retaining the ones fulfilling the selection criteria and the most recent ones; however, if they fulfilled the selection criteria and provided additional information on outcomes of interest, they were retained for these outcomes only. However, if the findings were concordant, we extracted and synthesized only the most recent report of that database, fitting all selection criteria. Disagreements were resolved by consultation with a third co-author (A.U.).
2.3
Data extraction
Data on studies, patients, and treatment characteristics were independently extracted by 2 authors (S.M. and A.P.). The following variables were extracted from the included studies: first author’s name, publication year, country and continent of research, study design, number of centers involved, number of patients enrolled, recruitment period, inclusion and exclusion criteria, history of bladder cancer (BCa), surgical approach (open, laparoscopic and robotic), type of bladder cuff excision, tumor characteristics (tumor location and multifocality, stage, tumor size, and grade), information of intravesical chemotherapy (molecule used, timing, and treatment duration), intravesical recurrence rate, and minor and major complication rates.
2.4
Risk of bias (RoB) assessment
Two authors (S.M. and A.P.) independently evaluated the study quality of nonrandomized studies with the ROBINS-I tool [ ]. We judged each bias domain and overall risk of bias as “Low,” “Moderate,” “Serious,” or “Critical” RoB. For Randomized Controlled Studies (RCTs), we used the ROBINS-II tool [ ]. The RoB level was judged as ‘‘low’’, ‘‘some concerns, or ‘‘high’’ risk. For prospective and retrospective single-arm studies, RoB was evaluated according to European Association of Urology guidelines for systematic reviews of case series [ ]. The RoB level, in this case, was judged as ‘‘low’’ or ‘‘high’’ risk. The possible confounders were considered by consensus of 2 authors from a literature review. Disagreements were resolved by consultation with a third co-author (A.U.).
2.5
Statistical analysis
We undertook an initial descriptive analysis of the studies and a pooled analysis evaluating the intravesical recurrence rates in different intravesical chemotherapy settings. The pooled rates represent the average from multiple studies weighted by the inverse of their sampling variances. We performed a meta-analysis to evaluate the intravesical recurrence rates, comparing groups with and without intravesical chemotherapy. Sub-analyses included comparisons between single and multiple intravesical chemotherapy treatments, as well as between intraoperative and postoperative intravesical chemotherapy. We performed a meta-analysis if at least 2 references provided data on an outcome. The Mantel-Haenszel method with odds ratios (ORs) and the corresponding confidence intervals (CIs) were used to determine the association between intravesical recurrence rates and different intravesical chemotherapy schemes. We used dichotomous data to calculate pooled ORs and corresponding 95% CIs. We used a random-effect model to calculate ORs due to the a priori risk of heterogeneity between the included studies. We assessed heterogeneity using the Cochrane’s Q test and quantified it using I 2 values. In the case of significant heterogeneity (Cochrane’s Q test P < 0.05), we attempted to investigate and explain the heterogeneity by sensitivity analyses [ ]. All statistical analyses were performed using Cochrane Collaboration Review Manager software (RevMan v.5.4; Cochrane Collaboration, Oxford, UK) and OpenMetaAnalyst software. Statistical significance was set at P < 0.05.
3
Results
3.1
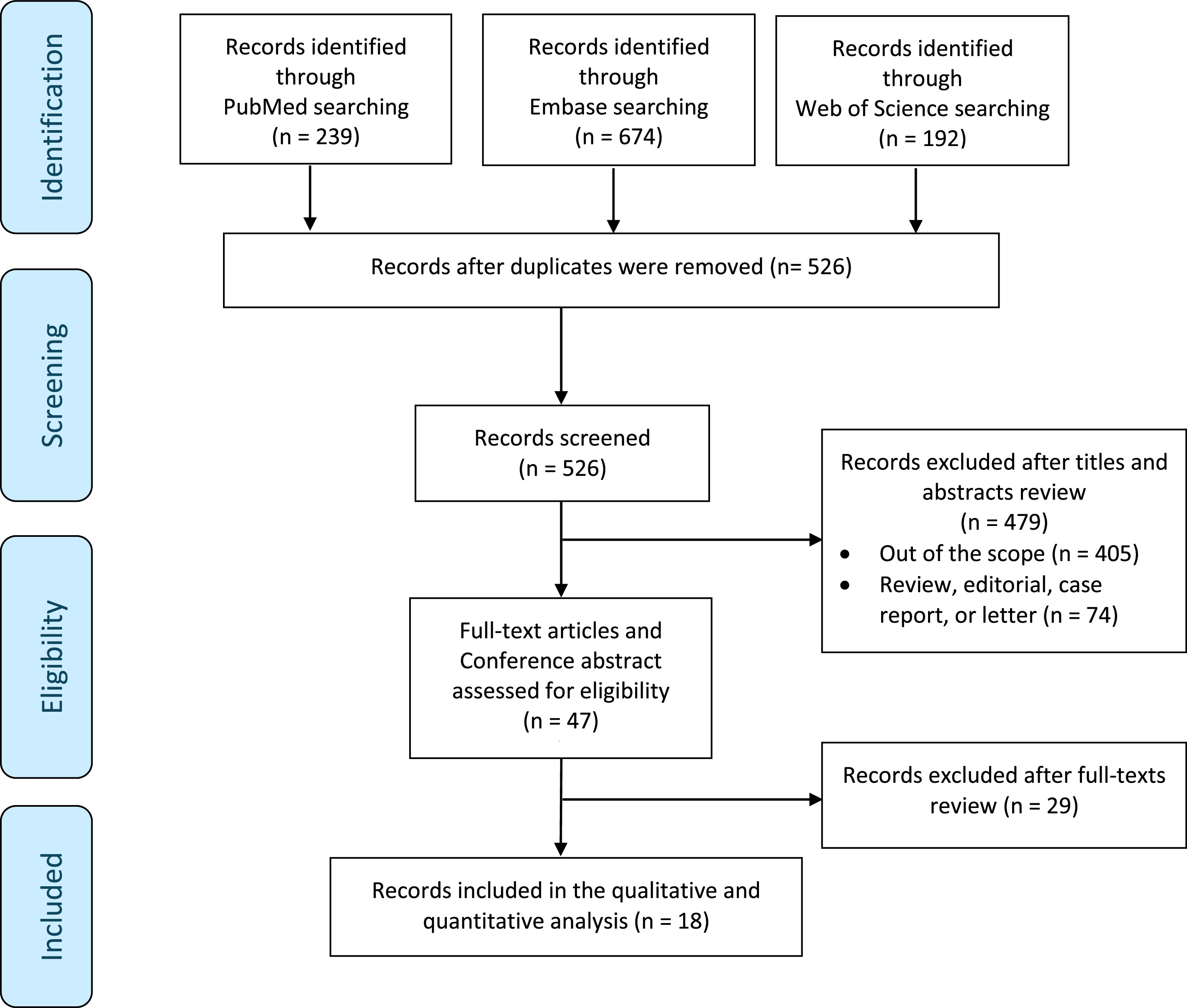
Study selection and characteristics
We summarized the study selection process in the PRISMA flowchart ( Fig. 1 ). A total of 1,105 records were identified. Of these, 47 full-text articles were assessed for eligibility, and 18 studies met our inclusion criteria [ , , , ]. Four studies were RCTs, twelve were nRCTs, and 2 were single-arm case series. The included studies were published between 2001 and 2024, and most were conducted using a retrospective design (14/18; 78%). In the Supplementary materials ( Appendix 2 ), we provided a complete description of the reasons for exclusion after full-text review. The qualitative and quantitative analysis involved 2,483 patients. Among them, 947 (38%) patients did not receive intravesical chemotherapy after RNU for UTUC. Of the patients who received intravesical chemotherapy, 1226 (49%) were treated with a single instillation and 310 (12%) with multiple instillations. Specifically, 250 (20%) patients received a single intraoperative instillation, 246 (20%) within 48 hours postsurgery, and 738 (60%) after 48 hours. Ten studies involved patients treated with MMC (459 patients), 8 with Epirubicin (881 patients), 2 with Gemcitabine (60 patients), and 2 with Pirarubicin (136 patients).
Eleven (61%) studies exclusively included patients with no prior history of bladder cancer (BCa), while 7 (39%) encompassed both patients with and without a history of BCa. Notably, two of these studies provided intravesical recurrence rates for both cohorts.
Table 1 shows the chemotherapeutic agents used and the timing of administration relative to RNU surgery, as well as the number of instillations and the rate of intravesical recurrence. A detailed description of the patients and tumor characteristics is provided in the Supplementary eTable 5 .
| Study, year | Study period | Country | Study design | IVC scheme | Surgical technique | N° of patients (IVC/no-IVC) | Stage ≥ 2 | HG | Multifocality | Previous BCa | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sakamoto, 2001 | 1993–1996 | Japan | RCT | 28 mg MMC+ 200 mg AraC (28 times over 2 yrs) vs. no-IVC | NR | 25 (13/12) | 0% | NR | 16% | 0% | 45.0 (6.0–65.0) |
| O’Brien, 2011 | 2000–2006 | UK | RCT | Single MMC 40 mg in 40 ml prior to removal of catheter vs. no-IVC | Open/VLS RNU with ‘‘rip and pluck’’ of the intravesical ureter | 239 (120/119) | 39% | 8% | 13% | 0% | 12.0 (NR) |
| Ito, 2013 | 2005–2008 | Japan | RCT | Single THP 30 mg for 30 min within 48 hrs after RNU vs. no-IVC | Open/VLS RNU + bladder cuff excision | 72 (36/36) | 46% | 45% | NR | 0% | 24.9 (2.6–39.3) |
| Harraz, 2019 | 2015–2017 | Egypt | RCT | Single EPI 50 mg for 30 minutes within 48 hrs vs. EPI 50 mg (6 weekly + monthly 1-yr maintenance) | Open/VLS RNU + bladder cuff excision | 74 (38/36) | 35% | NR | NR | 0% | SIC 18.5 (12.0–38.0) MIC 19.5 (12.0–36.0) |
| Yamashita, 2017 | 2012–2013 | Japan | Prospective | Single THP 30 mg for 30 min within 48 hrs after RNU vs. no-IVC | Open/VLS RNU + bladder cuff excision | 74 (11/63) | 59% | NR | NR | 0% | 24.0 (9.0–45.0) |
| Abe, 2009 | 1990–2004 | Japan | Retrospective | PEI vs. no-IVC | Open/VLS RNU + bladder cuff excision | 59 (21/38) | 60% | 21% | 26% | 0% | 50.5 (12.0–155.0) |
| Long, 2017 | 2004–2012 | China | Retrospective | Single postoperative EPI 30 mg for at least 30 min within 1–2 weeks + EPI 30 mg 6–8 times weekly vs. no-IVC | Open RNU + open bladder cuff excision or pluck technique | 685 (220/192/273) | 64% | NR | 25% | NR | 47 (36–62) |
| Huang, 2019 | 2000–2016 | China | Retrospective | Single postoperative EPI 30–50 mg or THP 30–50 mg or MMC 20–40 mg for at least 30 minutes within 2 weeks vs. multiple postoperative IVC (at least 6 weekly doses) vs. no-IVC | Open/VLS RNU + bladder cuff excision | 270 (130/99/41) | 59% | 67% | NR | NR | 27.5 (10.8–52.8) |
| Freifeld, 2020 | 2012–2018 | USA | Retrospective | Intraoperative MMC 20–40 mg or GEM 1–2 g in 50 ml for 60 min (30−120) vs. postoperative IVC | RNU + bladder cuff excision | 137 (75/26/36) | 34% | 69% | 13% | 30% | 8.0 (1.0–55.0) |
| Fan, 2022 | 2006–2017 | China | Retrospective | Weekly EPI 30 mg in 50 ml vs. no-IVC | RNU + bladder cuff excision | 89 (41/48) | 78% | 74% | 23% | 0% | 29.2 (2.3–122.1) |
| Said, 2023 | 2016–2020 | USA | Retrospective | Perioperative GEM 2 g for 1 h (after bladder cuff closure) vs. MMC 40 mg or DOX 50 mg (for 1 h, drained before bladder opening) | Robotic RNU + bladder cuff excision | 55 (24/NA) | 36% | 71% | 29% | 40% | GEM: 11.9 (6.4–20) dIVC:19.6 (12.2–30.5) |
| Long, 2016 | 2004–2012 | China | Retrospective | Single postoperative EPI (30 mg retained for at least 30 min within 1/2 wks) vs. no-IVC | Open/VLS + bladder cuff excision or pluck technique | 320 (161/159) | 66% | NR | 25% | NR | 52.0 (38.0–68.0) |
| Jiang, 2017 | 2005–2015 | China | Retrospective | Postoperative THP (8 weekly + 6–10 monthly) vs. no-IVC | Open RNU + bladder cuff excision | 110 (50/60) | 45% | 69% | 14% | 9% | 29.0 (2.0–113.0) |
| Noenning 2018 | 2008–2016 | USA | Retrospective | Intraoperative MMC 40 mg in 40 ml for 30–45 min with drainage prior to bladder cuff removal vs. postoperative (day 1) MMC 40 mg in 40 ml | Open/VLS/robotic RNU + bladder cuff excision | 51 (30/21) | 37% | 69% | NR | 37% | I: 22.0 (NR) P: 12.0 (NR) |
| Gulamhusein, 2020 | 2017–2019 | UK | Retrospective | Postoperative MMC 40 mg in 40 ml within 48 h for 60 min vs. no-IVC | Robotic RNU + bladder cuff excision | 69 (69/NA) | NR | 55% | NR | 41% | 13 (2–30) |
| Nadler, 2021 | 2017–2020 | Denmark | Retrospective | Intraoperative MMC 40 mg in 40 ml for 1 h vs. no-IVC | Robotic RNU + bladder cuff excision | 62 (47/15) | 29% | 18% | NR | 37% | 16 (9–27) |
| Wijngaarden, 2023 | 2007–2021 | Netherlands | Retrospective | MMC 40 mg in 50 ml (6 weekly + 5 monthly) starting after 30 days vs. no-IVC | VLS RNU with no sutures in the bladder after cuff excision | 66 (39/27) | 15% | NR | NR | 8% | IVC: 110 (100–120) no-IVC: 48 (33–63) |
| Lee, 2024 | 2018–2022 | Korea | Retrospective | EPI 50 mg in 50 ml within 48h for 60 min vs. no-IVC | Robotic or open/VLS RNU + bladder cuff excision | 167 (51/116) | 55% | NR | NR | 0% | EPI: 19.6 (16.4–28.7) noIVC:24.8(18.3–34.7) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree