The role of reduced-intensity allogeneic hematopoietic stem cell transplantation (HSCT) from a variety of donor sources in improving survival for children with familial hemophagocytic lymphohistiocytosis (HLH) is well-documented. The heterogeneity of adult-onset HLH has complicated evaluation of initial therapy and of HSCT as definitive treatment. Therapy for adults with HLH is often individualized, but institutions are now generating algorithms that include HSCT based on growing experience. Consolidation of these data is needed to optimize management of the growing number of adults recognized to have HLH and to achieve dramatic improvements in survival.
Key points
- •
In cases of familial or relapsed/refractory HLH, hematopoietic stem cell transplant (HSCT) is indicated for optimal survival.
- •
Seventy-one percent of patients with pediatric/familial HLH for whom transplant is indicated are able to undergo HSCT. Long-term survival of children who undergo transplant is 66%.
- •
Use of alemtuzumab before conditioning favorably impacts donor chimerism and is becoming standard peritransplant therapy.
- •
Adult-onset HLH is increasingly recognized, and patients often bear classical familial HLH-associated genetic variants.
- •
The role of HSCT in adults, particularly older adults, is unclear, but overall outcomes after HSCT are encouraging.
Introduction
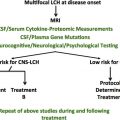
Hemophagocytic lymphohistiocytosis (HLH) was initially described as an inflammatory condition affecting young children with an abysmal prognosis. Early introduction of chemotherapeutic and immunomodulatory agents to suppress the unbridled yet ineffective phagocytic, natural killer (NK), and T-cell activity of HLH through the HLH-94 protocol had a dramatic impact on survival. To consolidate disease remission, children with familial, genetically based HLH and those with relapsed or primary refractory HLH require allogeneic hematopoietic stem cell transplantation (HSCT). Introduction of alemtuzumab into the peritransplant regimen is under investigation to improve chimerism and other clinical outcomes. Unfortunately, the role of stem cell transplantation in adults with HLH is not as clear-cut. HLH often presents in adults in concert with an underlying malignancy and may shift the decision to pursue allogeneic HSCT sooner in the cancer treatment algorithm. When adults present with HLH without an accompanying lymphoma or leukemia, decisions to pursue HSCT are individualized and, unfortunately, not informed by adequate published data. At our institution, this decision is made based on the constellation of underlying mutations/genetic variants, aspects of the HLH remission after therapy, and donor availability.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) was initially described as an inflammatory condition affecting young children with an abysmal prognosis. Early introduction of chemotherapeutic and immunomodulatory agents to suppress the unbridled yet ineffective phagocytic, natural killer (NK), and T-cell activity of HLH through the HLH-94 protocol had a dramatic impact on survival. To consolidate disease remission, children with familial, genetically based HLH and those with relapsed or primary refractory HLH require allogeneic hematopoietic stem cell transplantation (HSCT). Introduction of alemtuzumab into the peritransplant regimen is under investigation to improve chimerism and other clinical outcomes. Unfortunately, the role of stem cell transplantation in adults with HLH is not as clear-cut. HLH often presents in adults in concert with an underlying malignancy and may shift the decision to pursue allogeneic HSCT sooner in the cancer treatment algorithm. When adults present with HLH without an accompanying lymphoma or leukemia, decisions to pursue HSCT are individualized and, unfortunately, not informed by adequate published data. At our institution, this decision is made based on the constellation of underlying mutations/genetic variants, aspects of the HLH remission after therapy, and donor availability.
Achieving remission in pediatric hemophagocytic lymphohistiocytosis
Familial HLH was initially described by Farquhar and Claireaux in two infant siblings who both had a rapidly fatal clinical course. Over the succeeding decades, genetic underpinnings of the disease in familial cases were described, namely autosomal-recessive mutations at 9q21.3-locus 6 and in perforin, MUNC 13–4, Syntaxin 11, and Syntaxin Binding Protein 2/MUNC 18 to two genes. These mutations affect cytotoxic granule composition, transport, and release. They result in impaired apoptosis and a vicious cytokine-driven, cell-mediated inflammatory response. Provoking infectious agents, particularly those of the herpes virus family, were identified. Lymphocyte-directed chemotherapy and immunotherapy were noted to have some efficacy in small studies, but children with familial HLH all experienced relapse.
The Histiocyte Society’s prospective international therapeutic study, HLH-94, represented the first large effort to systematically define the disease and implement a standardized therapeutic strategy. On this study, children younger than 15 years of age were treated with 8 weeks of induction therapy consisting of tapering dexamethasone doses, etoposide, and intrathecal methotrexate. Patients with no evidence of familial disease who showed disease resolution after the 8-week induction period were followed but did not continue to further therapy unless reactivation occurred. For patients with familial, clinically persistent, or relapsing disease, continuation therapy consisting of dexamethasone pulses, etoposide doses every 2 weeks, and cyclosporine was recommended. Allogeneic HSCT was pursued in those patients for whom a suitable donor was available.
Long-term results of this study, representing a cohort of 227 patients with greater than or equal to 5 years follow-up, demonstrated that 86% of patients were alive after the 8-week induction course; 59% of these had no signs of active disease. Children who did not survive the induction period were more likely to have presented with hyperbilirubinemia, renal failure, and abnormal findings on brain imaging. Notable characteristics of patients in this study included a median age of 8 months; 76% were younger than 2 years of age. Neurologic symptoms were present in 33% before therapy, and 46% had a history of recent infection. Familial disease was documented in 24%. Poor prognoses were associated with neurologic symptoms and central nervous system (CNS) pleocytosis at presentation and age less than 6 months. In this and subsequent studies, thrombocytopenia, initial or persistent ferritin levels greater than 2000 ng/mL, degree of soluble interleukin-2 receptor (sIL-2R) elevation, and the rate of decline of ferritin had prognostic implications. A ferritin decrease of less than 50% imparts an odds ratio for death of 17 when compared with a ferritin decrease of greater than 95% during therapy. Therapeutic guidelines and diagnostic criteria were further updated in the HLH-2004 treatment protocol, with primary changes being initiation of cyclosporine at the start of induction and specific guidelines on management of CNS disease. An alternative induction regimen consisting of steroids, cyclosporine, and antithymocyte globulin (ATG) instead of etoposide has been investigated. A higher initial complete response rate of 73% was seen but so was a 25% rate of early relapse and death before subsequent therapies.
For patients with either primary refractory or relapsing disease, multiple salvage therapies have been investigated. The best quantity and quality of data supports use of alemtuzumab, an antibody capable of rapidly and efficiently eliminating CD52-expressing cells, which include most mononuclear subsets (B-cell, T-cell, and NK cell lymphocytes, monocytes, macrophages, monocyte-derived dendritic cells, and eosinophils) but not hematopoietic stem cells. Building on case reports, a retrospective analysis of 22 children and adults with refractory HLH manifested by elevated ferritin and sIL-2R levels, cytopenias, organomegaly, and/or continued hemophagocytosis was conducted. Alemtuzumab was introduced in dose-escalated, fixed dose, or individualized schema, either intravenously or subcutaneously. The median alemtuzumab dose was 1 mg/kg (range, 0.1–8.9) over a median of 4 days (range, 2–10). Half of patients received subsequent additional courses of alemtuzumab. After 2 weeks, most patients experienced decreases in inflammatory markers: 67% of patients evaluable for ferritin had a greater than or equal to 25% decrease and 78% of patients evaluable for sIL-2R had a 1.6- to 4-fold decrease. Seventy-six percent of patients with neutropenia (ANC <2000 cells/μL) had increases in absolute neutrophil count (ANC). Liver function tests improved in all affected individuals. Unfortunately, no patient experienced a complete response to alemtuzumab therapy. There was no correlation of response with identification of a genetically predisposing mutation. Most patients went on to receive further definitive therapy. Nine of the 22 patients experienced bacteremia or candidemia, a third had cytomegalovirus viremia, a quarter had Epstein-Barr virus (EBV) viremia, and a quarter had adenovirus in the serum in the first few months following alemtuzumab therapy. Seventy-seven percent of patients survived to undergo HSCT.
Evidence for using other anti-inflammatory agents to block cytokines, macrophages, and/or T cells is based on case reports. Therapies have been successful in secondary HLH and macrophage activation syndrome arising out of rheumatologic disease using infliximab (anti–tumor necrosis factor-α), anakinra (anti–IL-1 receptor), tocilizumab (anti–IL-6), and daclizumab (anti-CD25). Success in these cases was defined as response of symptoms, tolerating a steroid taper, or survival to definitive therapy. Splenectomy has also been investigated. Long-term outcomes after these therapies followed by subsequent HSCT have not been studied in a systematic fashion.
Rationale for allogeneic hematopoietic stem cell transplantation in pediatric hemophagocytic lymphohistiocytosis
Although there have been case reports of HSCT for HLH since 1986, the first series examining its use was published in 1991 in nine children with “familial HLH” based on their age of presentation. They were treated initially with etoposide, steroids, and intrathecal methotrexate. Of 22 patients enrolled, 16 survived induction therapy, and 15 entered complete remission (CR). Ten of the patients in CR received maintenance chemotherapy. Six patients (five in CR, one in partial remission [PR]) underwent upfront stem cell transplant. Five had an HLA-matched sibling donor; one had a single antigen-mismatched parent as donor. Three additional children who relapsed on chemotherapy subsequently underwent HSCT from a two- or three-antigen mismatched parental donor while in PR. Conditioning consisted of a myeloablative regimen of cyclophosphamide, busulfan, and etoposide/VP-16 (CBV). All patients received bone marrow–derived stem cells. Notable findings were that 8 of 10 patients on maintenance chemotherapy relapsed in a mean of 5.4 months (range, 2–8 months), including four in the CNS. The two patients surviving after chemotherapy alone had no documented family history of HLH. Of the five children who underwent HSCT from a matched related donor (MRD), four were alive without therapy at more than 1 to 6 years. The recipient who relapsed had received stem cells from a sister who subsequently developed HLH herself. Of the four patients transplanted from non-HLA identical donors, one did not engraft and the other three had active disease at the time of transplant. All died of HLH progression. Restoration of normal NK cell activity after HSCT was seen in those with prolonged survival. This study established two tenets in treatment of pediatric HLH: chemotherapy alone is not sufficient for long-term control of familial HLH; and HSCT from a sibling donor improves survival for familial HLH. Other questions raised and under investigation in current trials were (1) what is the role of unrelated and mismatched donors in HLH, (2) how important is donor chimerism in maintaining remission after HSCT, (3) how crucial is disease activity at time of HSCT, and (4) how might inherited risk factors for HLH in an asymptomatic sibling donor impact prognosis in the recipient.
Confirming these observations, 48 children, 33 of whom had proven familial HLH and 15 with relapsed or refractory HLH, underwent HSCT after induction therapy with HLH-94 etoposide-based therapy or steroids, cyclosporine ± ATG. A total of 56% were in CR, 34% in PR, and 10% had active disease at the time of HSCT. Fourteen had matched sibling donors and received T-cell replete marrows. The others received T-cell depleted transplants, four from matched unrelated donors (MUD), one from a two-antigen mismatched donor, and 29 from related haploidentical donors. Conditioning entailed myeloablative CBV or cyclophosphamide, busulfan, and ATG. Event-free survival was 58.5% with median follow-up of more than 5 years. Donor compatibility in this study had no significant impact on survival, except when HLH was active at transplant, in which case MRD and MUD recipients had improved outcomes ( P = .03). There was a trend to worsened survival with uncontrolled HLH at time of HSCT ( P = .053).
Several additional observations arose from this trial. One was a high rate of both primary graft failure and secondary graft rejection affecting 25% of patients. Active HLH at time of HSCT, older age of the recipient, and perhaps having a haploidentical donor were associated with primary graft failure. Twelve of the 15 patients with graft failure underwent a second transplant. The second was that HLH recurrence was the primary cause of death in 50% of cases. In 28 long-term survivors, 50% had full donor chimerism and 50% had mixed chimerism (<95% donor). When donor chimerism was greater than 10% to 20%, stable CR of HLH was maintained.
In the largest study of HLH pediatric patients to date involving 249 patients enrolled on HLH-94, 14% died during the initial induction period, primarily from active disease. Overall survival (OS) at 5 years was 54%. One hundred twenty-four patients underwent HSCT, primarily with myeloablative CBV conditioning ± ATG. Five-year survival after HSCT was 66%. There was a trend to improved 5-year survival in patients with CR at HSCT (72%) versus those with active HLH (58%; P = .064). A total of 53 patients survived without HSCT. These patients were older (median age, 24 months); female; and less likely to have hepatomegaly, splenomegaly, persisting hyperferritinemia, or neurologic complications. Fifty-seven percent of patients surviving without HSCT were from Japan, and 52% had an infectious trigger, primarily EBV. None of those with familial HLH survived without HSCT.
Advances in conditioning intensity
In early studies of HSCT for HLH, toxicities with a myeloablative conditioning (MAC) regimen, usually CBV ± ATG, were significant. Small series investigated substituting total body irradiation for etoposide. Toxicities included significant rates of veno-occlusive disease (28%–38%) particularly in haploidentical transplants, after ATG, and in patients younger than 12 months. Rates of significant infections, especially viral infections, were 60% to 72%. Rates of acute graft-versus-host disease (GvHD) ranged from 17% to 44%; rate of chronic GvHD was 9%. In HLH-94, 29 of the 42 deaths after HSCT occurred in the first 100 days, and transplant-related mortality (TRM) affected 23% of recipients. In one study, 50% of children required intensive care unit admission after MAC HSCT. Long-term survival ranged from 45% to 65%.
These rates of complications were higher than expected or acceptable in pediatric transplants, so reduced-intensity conditioning (RIC) regimens were investigated to decrease TRM while still resetting the immune dysregulation of HLH. Cooper and colleagues first described 12 children who underwent RIC (primarily fludarabine, melphalan ± busulphan) and established feasibility. All patients in this series engrafted. Survival at a median of 30 months was 75% with all patients in CR, despite one-third of survivors having mixed chimerism. Rates of TRM, acute GvHD, and chronic GvHD were 25%, 33%, and 25%, respectively. In a subsequent update on a total of 25 patients, survival rates were 84% at a median of 3 years after HSCT. Similar results were seen among 40 patients with familial HLH at Cincinnati Children’s Hospital, 14 with MAC (CB + ATG ± etoposide) and 26 with RIC consisting of fludarabine, melphalan, and alemtuzumab. Approximately 60% in each group were in CR at time of HSCT with a substantial portion (35%) of RIC patients having undergone salvage therapy with alemtuzumab before HSCT. Most patients received bone marrow stem cells from unrelated fully matched or single antigen-mismatched donors. Two patients received umbilical cord stem cells. Estimated 3-year survival was 43% after MAC and 92% after RIC ( P = .0001). All patients engrafted. Rates of bacterial infections were 14% in MAC and 15% in RIC; rates of EBV, cytomegalovirus, adenoviral, and other viral infections/viremia were 29%, 29%, 15%, and 29% in MAC and 15%, 27%, 38%, and 8% in RIC, respectively. Grades II and III acute GvHD rates were 14% in MAC and 8% in RIC, without any grade IV acute GvHD seen. Chronic GvHD was not seen after MAC; 12% of RIC patients experienced limited chronic GvHD. Although not all studies replicate these findings of improved survival after RIC versus MAC regimens for HLH, the previously mentioned data have made HSCT with RIC the standard approach to familial, refractory, and relapsed pediatric HLH. Specific chemotherapy and immunosuppression agents, such as fludarabine, melphalan, treosulfan, ATG, and alemtuzumab, have all been used in conditioning with similar success.
Use of alternative donor sources
Given the familial clustering of HLH in pediatric cases, unrelated, mismatched, haploidentical, and umbilical cord donors have always been the main stem cell sources for HSCT. (Development of disease in initially unaffected sibling donors can be seen.) In the HLH-94 study, among 124 HSCT recipients, 5-year survival rates were 74% for MRD, 76% for MUD, 61% for mismatched unrelated donors (MMUD), 43% for haploidentical donors, and 80% for the 10 umbilical cord recipients. Survival between MRD and MUD recipients was not significantly different. In a separate analysis of 86 children receiving HLH-94 followed by MAC HSCT, adjusted odds ratio for mortality were 1.93 (confidence interval, 0.61–6.19) for MUD, 3.31 (confidence interval, 1.02–10.76) for haploidentical donors, and 3.01 (confidence interval, 0.91–9.97) for MMUD when compared with MRD. These trends have been recapitulated in smaller series. Haploidentical donor sources were often associated with increased risk of graft failure.
Experience with umbilical cord blood (UCB) stem cells has recently grown. Ohga and colleagues found survival greater than 65% after cord transplant in 28 patients, both those with familial and EBV-triggered HLH; those patients received primarily MAC. In that study, the risk of death for familial HLH patients was marginally higher for those receiving cord ( P = .07) versus MUD stem cells. Rates of engraftment were 60% and similar between stem cell sources. A recent study of 13 familial HLH patients confirmed the feasibility of RIC UCB transplants for HLH. Ten patients showed initial engraftment, two more engrafted after the second cord transplant, two had late graft failure, and two relapsed with HLH. Thus, although not as well studied, UCB transplant seems feasible with outcomes similar to transplants from other unrelated stem cell sources.
Specific role of alemtuzumab and import of post–hematopoietic stem cell transplantation chimerism
Alemtuzumab has been used in many stages of HLH, as salvage therapy and in HSCT conditioning. Studies of alemtuzumab demonstrate clear efficacy in patients with refractory disease with a 64% response rate and 77% rate of survival to HSCT. All but one patient undergoing HSCT survived to Day 100 with an overall probability of long-term survival of 64%. Thus, in patients surviving the salvage regimen, outcome after HSCT is similar to those undergoing upfront transplantation.
Alemtuzumab has been successfully incorporated into RIC in place of ATG. But there is higher prevalence of persistent donor chimerism. Among 26 patients receiving fludarabine, melphalan, and alemtuzumab pre-HSCT, all engrafted but 65% showed mixed chimerism. Ouachée-Chardin and coworkers demonstrated that CR was sustained as long as donor chimerism remained greater than 10% to 20% and patients did not experience secondary graft rejection. Six of 21 survivors after HSCT in the study by Cooper and colleagues had mixed chimerism. All retained their grafts and stayed in remission, including one who had donor cells only in the T-cell compartment. The pathophysiology of early HLH recurrence in situations of waning chimerism is unknown, but some have cited persistence of host macrophages, NK cells, and T cells in combination with high incidence of viral reactivation early after HSCT as factors enabling recurrent immune dysregulation.
Given the high incidence of mixed chimerism with alemtuzumab and resulting concern for increased relapse, several interventions are used. Donor lymphocyte infusion (DLI) may be pursued when chimerism rapidly declines or drops below 40% to 60% within the first 6 months after HSCT. Marsh and colleagues administered DLI (one to three doses) and/or CD34 + stem cell boost to 14 of 17 patients with mixed chimerism after RIC HSCT. Five patients developed grade II or III acute GvHD after DLI. One patient who dropped to 9% donor chimerism experienced HLH recurrence. The rate of mixed chimerism was decreased (29% vs 79%; P = .0225) when alemtuzumab was delivered at higher dose farther before or “distal” to transplant (Days -22 to -19) as opposed to lower dose “proximal” administration (Days -12/8 to -9/4). However, acute GvHD was increased with distal dosing. To follow up this observation, an intermediate schedule of alemtuzumab dosed Day -14 to Day -10 before HSCT to a total of 1 mg/kg was compared retrospectively with proximal and distal administration involving several dosing schedules along with fludarabine and melphalan conditioning. The 71 patients were children and young adults up to age 26; 43% had documented genetic diagnoses and an additional 6% were single heterozygotes for classical mutations. Seventy-six percent of patients received fully matched stem cells, 24% had MRDs, and almost all received bone marrow. With the caveat that a variety of doses and schedules were used within the proximal and distal cohorts, the intermediate group demonstrated reduced rates of mixed chimerism (31%) compared with the proximal group (72%; P <.01) and distal group receiving greater than or equal to 2 mg/kg alemtuzumab (75%; P = .04). Regarding dynamics of chimerism, only 13% in the intermediate alemtuzumab group dropped below 20% as compared with 25% of patients in the proximal and distal high-dose groups. Interventions pursued when donor chimerism dropped to less than 95% were withdrawal of immune suppression, followed by one or more DLIs at rates of 14%, 53% ( P = .01), and 38% ( P = .02) in the intermediate, proximal, and high-dose distal groups, respectively. These interventions resulted in more than 75% of patients maintaining more than 50% donor chimerism at last follow-up; four patients required a second allogeneic HSCT. Rates of grades II to IV acute GvHD after initial transplant were low at 0%, 4%, and 13% in the intermediate, proximal, and distal groups, respectively, and 8% to 14% after withdrawal of immune suppression ± DLI. In multivariable analysis, there were no significant differences in survival (80%–91% at 1 year and 80%–82% at last follow-up) based on alemtuzumab dosing schedule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree