Although numbers up to 56 are included in Table 5.1, some are omitted: 13–16, because they were given to RhA, etc., no longer regarded as distinct antigens; and 25, because it was allotted to LW, now known to belong to a system independent of Rh and 38 (Duclos) because this antigen is now known to be part of the RHAG system (Daniels et al. 2004, 2009).
Of the antigens listed in Table 5.1, Rh 9, 10, 11, 20, 22, 23, 28, 30, 32, 33, 35, 36, 37, 40, 42, 43, 45, 48, 49, 50, 52, 53, 54, 55, 56 and 57 are found in fewer than 1% of white people; Rh 17, 29, 34, 39, 44, 46, 47 and 51 are found in more than 99%. A list of all antigens currently assigned to blood group systems can be found at http://www.isbtweb.org/workingparties/red-cell-immunogenetics-and-blood-group-terminology/blood-group-terminology.
Wiener’s Nomenclature (Rh-hr)
Although this nomenclature is almost obsolete, some short symbols based upon it are still in use; those for antigens (seldom used) are included in Table 5.1 and those for haplotypes (quite frequently used) are in Table 5.2.
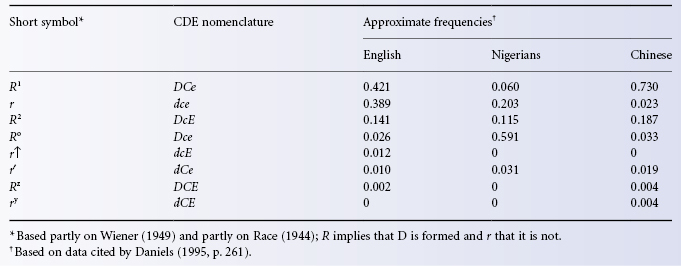
Table 5.2 Approximate frequencies of common haplotypes in selected populations.
Rh Genes
In 1943, RA Fisher proposed that there were three closely linked genes, Cc, Dd and Ee, which determined corresponding antithetical antigens (Race 1944) and he later proposed that the order of the genes was DCE (Fisher and Race 1946). Experience soon showed that d did not occur and it was presumed that d was an amorphic allele. Application of the techniques of molecular biology has since shown that there are only two genes: D, which has no allele and a second gene, CeEe (Colin et al. 1991), which has many alleles. It is convenient to use d to indicate the absence of D. Further details of the genetics of Rh and of the molecular biology of Rh antigens are given in a later section.
Rh Phenotypes
The completeness with which the Rh phenotype can be determined depends on the antisera available; if anti-c is available but not anti-C, samples can be classified as c positive (i.e. cc or Cc) and c negative (i.e. CC). If anti-C is also available, Cc can be distinguished from cc.
A convenient notation for Rh phenotypes is that introduced by Mourant (1949). Suppose a sample is tested with anti-D, anti-C, anti-c and anti-E and gives positive reactions with all four antisera: the phenotype is written DCcE. If positive reactions are obtained with anti-D, anti-C and anti-c, but the reaction with anti-E is negative, the phenotype is written as DCcee, as an absence of E implies a double dose of e. Similarly, red cells that fail to react with anti-D are described as dd. Mourant’s notation is occasionally misleading; for example, although a negative reaction with anti-E usually implies that the cells are ee, they may be ese. Perhaps a more important objection to the notation is that it is very clumsy in speech; for this reason, the short symbols shown in Table 5.2 are often used for both phenotypes and genotypes.
Phenotypes are often symbolized as the most probable genotype. For example, use of the term R1r to represent the phenotype DEcee implies that the genotype is R1r (DCe/dce) but it may be R1R0 (DCe/Dce). When a given blood sample is described in this way, the symbols should not be italicized, as they do not describe true genotypes.
One of the advantages of the numbered nomenclature is that the sera used in testing a sample are always indicated. Thus the description Rh: −1, −2, −3 indicates that the sample does not react with anti-D, anti-C and anti-E.
Determination of the Genotype
When a woman has anti-D in her serum it is important to know whether her partner is Dd or DD. If he is DD he can father only D-positive offspring but if he is Dd there will be a 50% chance that any child which he fathers will be D negative and so be unaffected by anti-D in his/her mother’s plasma. Routine serological tests do not distinguish reliably between red cells of DD and Dd individuals; indeed, there is an overlap between the numbers of antigen sites on the cells of the two genotypes. If relatives are available for serological testing, it may be possible to establish the genotype of a D-positive subject with certainty. For example, anyone with a D-negative parent cannot be DD.
A variety of molecular methods for the determination of the Rh D genotype of D-positive subjects have been described and are discussed in Chapter 12 (reviewed in Van der Schoot et al. 2003).
Common Rh Genotypes and Phenotypes
As Table 5.1 shows, in white people the commonest Rh haplotypes are DCe and dce and the commonest three genotypes are thus (1) DCe/dce with an approximate frequency of (0.42 × 0.39) × 2 = 0.32, or 32%: the × 2 is accounted for by the fact that DCe can combine with dce and dce with DCe; (2) Dce/DCe with a frequency of (0.42 × 0.42) = 0.18, or 18%; and (3) dce/dce with a frequency of (0.39 × 0.39) = 0.15, or 15%. D negatives comprise dCe/dce, dCE/dce, etc., as well as dce/dce and total 17%.
The frequencies given in the foregoing paragraph are for an English population, in whom the overall frequency of the phenotype DCcee is about 35%; the approximate frequencies of the next commonest phenotypes in order are: DCCee 18.5%; ddccee 15%; DCcEe 13.5% and DccEe 11.5%. These together account for approximately 94% of the total Rh phenotypes of English white people (Race et al. 1948a). Phenotype frequencies in most other European white people are similar, although in Basques 20–40% of the population are D negative. The frequency of D negatives is only 0–1% in Burmese, Chinese, Japanese, Maoris, Melanesians, American Indians and Inuit, (Mourant et al. 1976).
Numbers of D Sites on Red Cells of Different Phenotypes
Using polyclonal IgG anti-D, followed by purified 125I-labelled anti-IgG, the numbers of available D antigen sites on intact red cells of various phenotypes (probable genotypes in parentheses) were as follows (Rochna and Hughes-Jones 1965):
- DCcee (DCe/dce) 9900–14 600;
- Dccee (Dce/dce) 12 000–20 000;
- DccEe (DcE/dce) 14 000–16 000;
- DCCee (DCe/DCe) 14 500–19 300;
- DCcEe (DCe/DcE) 23 000–31 000;
- DccEE (DcE/DcE) 15 800–33 300.
Similar figures, also using polyclonal IgG anti-D, have been published by others (Edgington 1971; Masouredis et al. 1976). Although estimates with monoclonal IgG anti-D have given broadly similar results, a considerable variation has been found, depending on the particular antibody used: some examples gave 10 000–12 000 sites, others 25 000–30 000 sites with yet others giving intermediate values (Gorick et al. 1988).
Four examples of D− −/D− − red cells were found to have between 110 000 and 202 000 sites per cell (Hughes-Jones et al. 1971) and a sample of D• •/D• • cells was found to have 56 000 sites per cell (Contreras et al. 1979).
Numbers of c, e and E Antigen Sites Per Red Cell
In one investigation, the figures were as follows (Hughes-Jones et al. 1971):
- c sites: cc cells, 70 000–85 000; cC cells, 37 000–53 000;
- e sites: ee cells, 18 200–24 000; eE cells, 13 400–14 500;
- E sites: 450–25 600, depending on the source of anti-E and the red cell phenotype.
In another investigation, using different methodology but also using polyclonal IgG antibodies, substantially different figures (for various probable genotypes) were obtained (Masouredis et al. 1976):
- c sites: dce/dce cells, 31 500; DcE/DcE cells, 24 000;
- e sites: DCe/DCwE cells, 20 000;
- E sites: DcE/DcE cells, 27 500; DcE/dce cells, 17 900.
Results with single examples of IgG monoclonal anti-c and anti-E gave the following results: on DcE/DcE cells, c sites 32 000 and E sites 38 000 (Bloy et al. 1988).
Quantitative Binding Studies Using Monoclonal Antibodies to Rh Proteins
Quantitative binding studies with radioiodinated murine monoclonal antibodies (R6A-type) reactive with red cells of normal Rh phenotype but not with Rhnull red cells identified 100 000–200 000 binding sites on normal red cells (Gardner et al. 1991). Monoclonal antibodies reactive with the Rh-related glycoprotein RhAG (2D10 type, Mallinson et al. 1990) see a similar number of binding sites (Gardner et al. 1991). These results suggest that data obtained with blood group antibodies specific for D, Cc and Ee antigens (see above) are the result of binding to a subset of the Rh polypeptides expressed at the red cell surface. It is known that the Cc and Ee antigens are encoded by the same gene and expressed on the same polypeptide (Smythe et al. 1996). Therefore, it might be anticipated that the number of Cc and Ee sites on a given cell would be the same. However, this assumes that the Cc and Ee antigens are equally accessible at the red cell surface and independent of one another. This is clearly not the case for C and E as expression of cDNA encoding CE antigens in the erythroid cell line K562 results in a cell surface Rh polypeptide reactive with anti-E, but poorly or not at all reactive with anti-C (Smythe and Anstee 2001).
Structure of Rh D, C, c, E and e
Rh polypeptides were first characterized biochemically by immune precipitation with Rh antibodies from intact red cells labelled with 125I. The radiolabelled Rh proteins were visualized by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography. The results revealed strongly labelled bands with an approximate molecular weight of 30 kDa (Gahmberg 1982; Moore et al. 1982). Subsequent studies indicated the presence of two polypeptides, one corresponding to the D polypeptide and the other to the CE polypeptide. Isolation and sequencing of cDNA encoding these polypeptides predicted that they encoded proteins of 417 amino acids, from which the translation-initiating methionine is post-translationally cleaved to give 416 amino acids in the mature protein (Le Van Kim et al. 1992; Anstee and Tanner 1993). These proteins lacked N-glycosylation sites and had a calculated molecular weight of 45.5 kDa. It is believed that the lower estimate for molecular weight (30 kDa) mentioned above, derived from mobility by SDS-PAGE, was aberrant because of anomalous binding of Rh polypeptide to SDS (Agre and Cartron 1991).
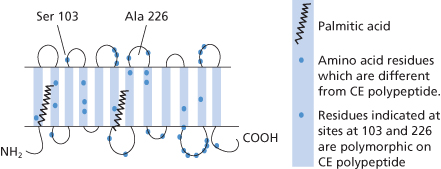
Hydropathy plots indicated that D and CE polypeptides have 12 transmembrane domains with the amino and carboxyl termini in the cytoplasm (Anstee and Tanner 1993; compare with Colour Plate 3.1; Figure 5.1.
The D and CcEe antigens are carried by proteins that are distinct but with 92% homology. In all, the CE polypeptide differs from the D polypeptide by only 35/36 amino acid substitutions, suggesting that the corresponding genes have evolved by duplication of a common ancestor gene (Le Van Kim et al. 1992; Figure 5.1).
Both D and CE have 10 exons (Mouro et al. 1994). C and c differ by one nucleotide change in exon 1 and by 5 nucleotide changes in exon 2 (Colin et al. 1994). However, C/c polymorphism appears to depend primarily on a mutation at position 103 (in exon 2): serine determines C and proline c (Anstee and Mallinson 1994; see also Colin et al. 1994). E/e polymorphism is determined by a single amino acid substitution at position 226 (in exon 5): proline determines E and alanine, e (Mouro et al. 1993). Initially, it was believed that different splicing isoforms are transcribed from CE, which has four main alleles, Ce, CE, ce and cE, each of which is ‘read’ to produce a C/c and an E/e mRNA, which are translated into substantially different polypeptides (Mouro et al. 1993), However, expression of the D and CE genes in the K562 erythroid cell line demonstrated that Cc and Ee antigens are carried on the same protein (Smythe et al. 1996). When Rh polypeptides are immunoprecipitated, a structurally similar glycoprotein (known as Rh-related glycoprotein, RHAG) is coprecipitated with them (Moore and Green 1987). The crystal structure of a bacterial (Nitrosomonas europaea) homologue of RHAG which has 36% sequence identity with human RHAG has been solved (Lupo et al. 2007; Li et al. 2007). This structure allows for reliable modelling of the overall structure of human RHAG and because of the similarity between RHAG and D and CE polypeptides it also becomes possible to model the structure of D and CE polypeptides (Burton and Anstee 2008; Colour Plate 5.1). These models inform our understanding of the structure of D, Cc and Ee antigens, particularly when viewed in comparison with the structure of an antibody Fab fragment. It is apparent the antigen binding site on Fab is of approximately the same dimensions as the whole of the surface exposed regions of monomeric D and CE polypeptides (Colour Plate 5.2). This size relationship provides an explanation for the widely recorded occurrence of antibodies whose reactivity for Cc antigens is influenced by the Ee polymorphism and vice versa (see below, Daniels 2002; Smythe and Anstee 2001) and the complex interdependency of reaction patterns obtained with different monoclonal anti-Ds (Table 5.3). The models also indicate that the D and CE polypeptides are not likely to function as transport proteins (Burton and Anstee 2008), an observation consistent with the suggestion that the polypeptides perform a secondary function by facilitating the assembly of the band 3/RHAG gas exchange complex formed at the time of enucleation (Huang and Peng 2005).
Table 5.3 Division of monoclonal anti-Ds into reaction patterns using D variant red cells.
Source: Scott 2002. Reproduced with permission of Elsevier.
Fatty Acylation of Rh Polypeptides
The serological activity of Rh proteins depends on the presence of phospholipid (Green 1968; Hughes-Jones et al. 1975). Palmitic acid appears to be covalently attached to Rh polypeptides by thioester linkages onto free sulphydryls on certain cysteine residues within the molecule (De Vetten and Agre 1988). Mutation of these cysteine residues to alanine does not prevent expression of D polypeptide in K562 cells, but the resulting polypeptide has altered expression of some epitopes of D, suggesting that palmitoylation may be important for the correct folding of the polypeptide (Smythe and Anstee 2000).
Genetic Basis of the D-Negative Phenotype in Different Races
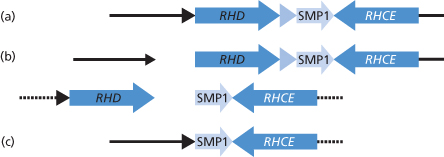
The organization of the RH genes was investigated in detail by Wagner and Flegel (2000). These authors reported that the D and CE genes are in opposite orientation on chromosome 1 (5′RHD3′–3′RHCE5′) with D centromeric of CE. The genes are separated by a stretch of around 30 kb, which includes another gene (SMP1). The D gene is flanked by two 9-kb regions of homology denoted rhesus boxes by Wagner and Flegel (Figure 5.2) and these authors suggest that the deletion of D, the common cause of the D-negative phenotype in white people, results from chromosomal misalignment at meiosis and subsequent unequal crossing over between the rhesus boxes (see Figure 5.2).
Figure 5.2 Structure of RH genes. (a) The physical structure of the RHD and RHCE gene locus. (b) An unequal crossing-over between the upstream and downstream Rhesus boxes can be triggered by their high homology. The breakpoint region in the Rhesus boxes was found to be of 100% homology for 903bp. (c) Resolving the crossed-over chromosome yields the RH gene structure of the extant RHD haplotype.
(Source: Wagner and Flegel 2000. Reproduced with permission of the American Society of Hematology.)
In black Africans the D-negative phenotype commonly results not from the absence of RHD but from inheritance of an altered RHD, which contains a duplicated 37-bp sequence comprising the last 19 nucleotides of intron 3, the first 18 nucleotides of exon 4 and a nonsense mutation in exon 6, which creates a stop signal (Tyr269stop). As a result of these changes, no D polypeptide reaches the surface of the red cell (Singleton et al. 2000). Of 82 D-negative black African samples studies by Singleton and colleagues, 67% had this altered RHD (referred to as the RHD pseudogene), 18% had a deletion of RHD and 15% had a hybrid gene (RHD–CE–Ds) in which exons 4–7 and the 3’ end of exon 3 of RHD are replaced by the corresponding regions of RHCE, an allele that produces no D antigen but is associated with expression of VS antigen, weak e and usually weak C. The frequencies of RHD deletion, RHD pseudogene and RHD-CE-Ds were 0.76, 0.21 and 0.45 respectively in 103 D negative blood samples from individuals living in central Congo analyzed by Touinssi et al. (2009).
The D-negative phenotype accounts for less than 1% of Asian individuals (see Table 5.2). In a study of 305 572 Chinese 0.46% (1401) typed as D negative (Yan et al. 2007). The very low frequency of the D-negative in Asian populations brings into question the necessity of routinely testing donors and patients for D in this region of the world. In one study from Taiwan discontinuing routine D typing over many years did not result in an increased incidence of anti-D (Lin, 2006). In a study of 294 D-negative Taiwanese, the most common cause of the D negative phenotype (185 individuals) was a deletion of RHD. Ninety-four individuals had the G1227A allele characteristic of the weak D phenotype denoted Del (see below for discussion of Del). In a study of 264 D-negative Koreans, 74% had a deletion of RHD, 9% had a hybrid RHD–CE–D and the remainder had the Del mutation G1227A in RHD. The very different molecular backgrounds of D negative phenotype in different racial groups become of considerable significance when DNA-based methods of D typing are contemplated. Clearly, a method that is very reliable in white people will not necessarily be reliable in other racial groups. It is essential to analyze the Rh genes of any given population in detail so that an appropriate molecular method can be devised for routine typing (see Chapter 12 for further discussion).
Weak D Antigens
A weakly reacting form of D was described as Du (Stratton 1946) and came to be considered as a definable phenotype. The original kind of Du was shown to be inherited (Stratton 1946; Race et al. 1948b).
The term Du is now redundant and has been replaced by the term weak D, which defines any D phenotype where the expression of D antigen is quantitatively weaker than normal (Agre et al. 1992). Weak D is distinguished from partial D, which defines a D phenotype qualitatively different from normal D. As red cells expressing qualitatively different D antigens may also give weak reactions with some anti-D reagents this whole area of blood grouping has been a source of great confusion over many years. Many examples of weak D and partial D have been examined at the DNA level (see below). This has allowed the correlation of sequence variation in RHD with topological models of the D polypeptide and led to the conclusion that mutations changing the amino acid sequence of D in regions of the protein predicted to be in either membrane-spanning domains or intracellular domains are a general feature of weak D, whereas mutations changing the amino acid sequence in regions of the protein predicted to be extracellular are a general feature of partial D (Wagner et al. 1999). It has been considered important to distinguish weak D from partial D in clinical practice because of the assumption that a weak D patient would not produce anti-D if transfused with D-positive blood (because the D antigen they express is weak but normal), whereas a partial D patient would have the potential to produce anti-D (against the part of D antigen they lack) and so should be given D- negative blood. However, the validity of this assumption is challenged by the demonstration of nucleotide substitutions in RHD encoding amino acid changes in different weak D samples (i.e. the D polypeptide is not normal) and by evidence that patients with weak D phenotype can produce allo-anti-D (Flegel et al. 2000). It is therefore a moot point as to whether or not distinguishing between weak D and partial D is of any practical value. It seems sensible to use a more general term like D variant and to subdivide this group on the basis of whether or not a given D variant antigen has stimulated anti-D of clinical significance. Comprehensive databases describing the molecular bases of the most common D variants in different populations are developing rapidly (see below). Ultimately, one can envisage the design of molecular methods to identify demonstrably immunogenic D variants that are common in a given population as an alternative, more reliable route to transfusion safety. Using flow cytometry, 35 samples classified as weak D were found to have at least 10 times lower expression of D than normal D-positive samples (Tazzari et al. 1994). Wagner and colleagues (2000a) examined 18 weak D types using flow cytometry and concluded that the number of D antigen sites varied from 70–4000 per red cell.
Current practice requires red cells from first-time donors to be tested for D using two potent agglutinating anti-D reagents and a sensitive automated method. Subsequent donations need to be confirmed with only a single potent anti-D. In a survey in which 15 000 samples from donors were tested in the Groupamatic, using potent anti-DC and anti-DE, the frequency of weak D, i.e. samples that were classified as D negative in the Groupamatic but which reacted with anti-D in the antiglobulin test, was 0.23% (Contreras and Knight 1989).
Del is a weak form of D common in Far Eastern populations detectable by demonstrating that anti-D can be adsorbed onto, and eluted from, red cells that do not give other positive serological reactions with anti-D (Okubo et al. 1984). In the Japanese population studied, some 10% of apparently D-negative samples were considered to be Del. In Hong Kong Chinese, the figure was about 30% (Mak et al. 1993). The molecular basis of Del is discussed in a later section.
Partial D Antigens (Categories of D)
The D antigen is unlike other blood group antigens as it comprises an entire polypeptide rather than structural changes within a polypeptide arising from single nucleotide substitutions (as is the case for most of the other blood group antigens, see Chapter 6). Because D and CE are encoded by two separate but highly homologous genes adjacent on the same chromosome (see later section) it is possible for exchange of DNA between the genes to occur during meiosis with the creation of hybrid genes encoding variant D polypeptides containing part of the normal D sequence replaced by CE polypeptide sequence. The red cells of individuals with such hybrid RHD may type as D positive because of the normal D sequence that is present, while at the same time making an antibody against normal D-positive red cells corresponding to the part of the D polypeptide that they lack. This antibody will have the specificity anti-D so the individuals will appear to be D positive with allo-anti-D. In the first study that recognized the existence of missing parts of D antigen, the red cells were described as Rh variants; originally, three, RhA, RhB and RhC were defined (Unger and Wiener 1959) and a fourth (RhD) was soon added (Sacks et al. 1959). The collection of original sera defining these four variants is no longer available.
In the second classification, D-positive subjects who have made anti-D were divided into seven categories (Tippett and Sanger 1962, 1977; Lomas et al. 1986). Antibodies made by different members of the same category may not be identical but, by definition, red cells and sera of members of the same category are mutually compatible. Several categories are characterized by having a particular low-incidence antigen, in addition to lacking certain parts of D. Classification by categories is likely to fall out of use eventually, because the sera used originally are scarce and rather weak (reviewed by Tippett et al. 1996).
A third classification became possible when large numbers of monoclonal anti-D reagents became available. In this classification different partial D antigens are distinguished by their pattern of reactivity with a large panel of monoclonal anti-Ds and allo-anti-D made by D-positive individuals is not employed. Using this approach, 30 different patterns of reactivity were observed (Table 5.3). This dramatic increase in the number of partial D phenotypes is a reflection of the experimental method (i.e. use of monoclonal antibodies), which allows detection of partial D in D-positive individuals who have not made allo-anti-D.
Structure of Weak D and Partial D Antigens
Once the structure of D and CE had been elucidated, Rh genes from individuals expressing different Rh blood group phenotypes could be sequenced in order to elucidate the molecular bases of the numerous Rh antigens. Essentially, two general mechanisms for generating antigenic diversity have been found, nucleotide substitutions and gene conversion. Nucleotide substitution resulting in a single amino acid change in the protein sequence is the commonest mechanism for generating antigenic change in all systems other than Rh and the MNS system (see Chapter 6). Rh and MNS differ from all the other systems in that the antigens are encoded by the products of two highly homologous adjacent genes (RHD/RHCE and GYPA/ GYPB respectively). The occurrence of two adjacent, highly homologous genes predisposes to misalignment between the genes when chromosomes pair at meiosis (for example, D with CE rather than D with D), a process which can result in the insertion or deletion of stretches of DNA sequence in the misaligned genes with the creation of novel DNA sequences, which, when translated, result in novel protein sequences and thereby novel antigens. This gene conversion mechanism explains why there are many more antigens in the RH and MNS blood group systems than in other blood group systems. Understanding the structure of Rh antigens is further complicated because different antigens encoded by RHD are referred to as partial D antigens, rather than having more distinctive names (see Table 5.3 and section above for discussion of partial D). In many cases, partial D antigens result from gene conversion events creating D polypeptides with substantial regions where D polypeptide sequence is replaced by CE polypeptide sequence. Many partial D phenotypes (DIIIa, DVa, DVI, DAR, DFR, DBT) have in common the substitution of sequence in exon 5 of RHD with sequence from exon 5 of RHCE. Exon 5 encodes that portion of the polypeptide predicted to form the fourth extracellular loop of the D polypeptide. Others (DIV) have substitution of sequence in exon 7 corresponding to the protein sequence predicted to form the sixth extracellular loop of D polypeptide (Figure 5.3). The molecular bases of red cells expressing weak D antigens have been studied by Wagner and colleagues (1999). Comprehensive databases listing the molecular bases of weak D and partial D antigens can be found at http://www.uni-ulm.de/-fwagner/RH/RB. In contrast with partial D antigens, where the genetic changes frequently involve exchange of large portions of D for CE and affect regions of the D polypeptide predicted to be exposed on the outside of the red cell, weak D generally derives from point mutations in RHD changing single amino acids in the D polypeptide. Of the many different weak D mutations described most, if not all, encode amino acid substitutions in the predicted transmembrane and cytosolic domains of the D polypeptide (Figure 5.4). These amino acid substitutions frequently cause substantial changes in the protein sequence, for example by introduction of charged or bulky residues, and presumably impede the transport and assembly of the D polypeptide to the red cell membrane, hence weak expression of D.
Figure 5.3 Gene structure of D variants. Exons derived from D gene in black. Exons derived from CE gene in white.
(Source: Daniels 2002. Reproduced with permission of John Wiley & Sons Ltd.)
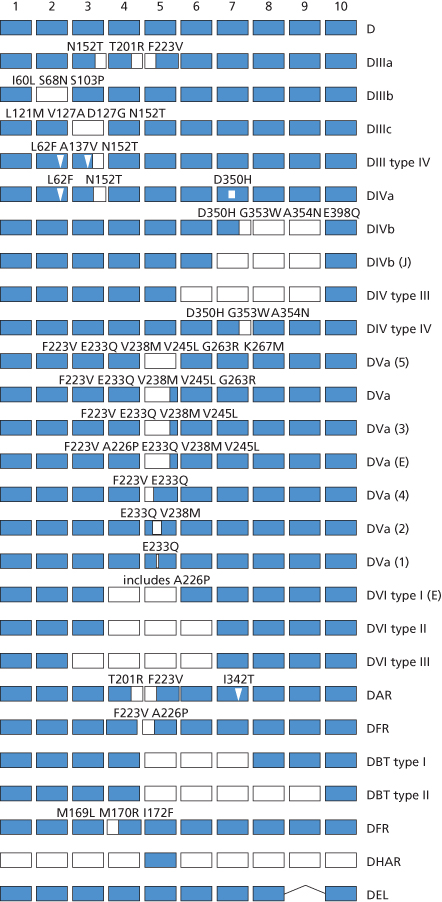
Figure 5.4 Weak D antigens. Position of amino acid substitutions associated with different weak D phenotype is indicated using the single letter code for amino acids with the wild-type amino acid on the left.
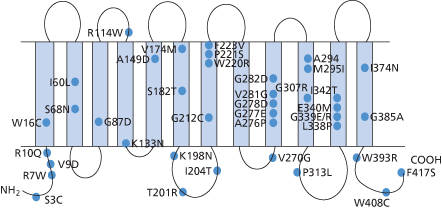
Clinical Relevance of D Variant (Partial D and Weak D) Phenotypes
The importance of determining whether a D variant phenotype is present on the red cells of a donor relates to whether or not the red cells will be immunogenic if transfused to a D-negative patient (or a patient with a different D variant). For a patient with a D variant phenotype the question is whether or not they will make anti-D if transfused with red cells of normal D phenotype. A study from Denmark concluded that serological typing for RhD detected all D variants in that population except DEL and that inadvertent immunization with D antigens was of the order of 1.4 in 100 000 D-transfusions (Krog et al. 2011).
D Variants as Recipients of Blood
Anti-Ds made in response to normal D positive blood by individuals expressing D variant antigens are not necessarily the same as anti-Ds made by D negative individuals and will not necessarily have the same clinical significance. Anti-D is defined by failure to react with D negative red cells which lack the entire D polypeptide or have a hybrid RHD-CE-Ds in which a large part of the Rh polypeptide encoded has CE sequence replacing D sequence (see above). In contrast, individuals with D variant phenotypes have a diverse array of mutations, some of which result from gene conversion events resulting in replacement of large (DVI) or small portions of D polypeptide sequence with sequence encoded by the CE (Figure 5.3; see discussion of DVI below and Colour Plate 5.3). Anti-D produced by a DVI individual is clinically significant since it has been the cause of haemolytic disease of the newborn in a DVI mother (HDN) (Lacey et al. 1983). Some result from single nucleotide transitions changing one or two amino acids in the D sequence predicted to be accessible at the extracellular surface of the mutant D polypeptide, for example DVa (Colour Plate 5.3). Others encode changes in sequence of D polypeptide in regions not predicted to be exposed at the red cell surface (Figure 5.3, see discussion of DAR below and Colour Plate 5.3).
Clearly, it cannot be assumed that all antibodies defined as anti-D by failure to react with D negative red cells have the same level of clinical significance. It can be predicted that anti-Ds made in response to normal D positive blood by individuals with D variants resulting from large insertions of CE sequence like DVI would make antibodies more comparable to that of D negative individuals, while those with D variants resulting from a single amino acid change at the cell surface would make anti-D more comparable in clinical significance to that of anti-C/c or anti-E/e. Antibodies made in response to amino acid sequence differences in regions of D polypeptide predicted to be in transmembrane or cytosolic regions (see discussion of DAR below) might be more comparable to anti-VS (Leu245Val) and have little clinical significance.
D Variants as Donors
Most monoclonal anti-Ds will type D variants as D positive (Table 5.3), thereby avoiding the possibility that D negative recipients will receive D variant blood. Exceptions are DVI which is relatively common in white people necessitating a policy of typing with two monoclonal anti-Ds one of which has been selected to detect DVI (see below) and Del where the expression of D antigen is too weak to be detected by agglutination methods (see below).
Common D Variants in White People
DVI is the most abundant serologically defined D variant occurring among weak D samples from white people. DVI is reported to constitute about 6–10% of weak D samples and has a phenotype frequency of 0.02–0.05% in white people (Leader et al. 1990; van Rhenen et al. 1994). Almost all subjects with the genotype DCe/dce have an antigen, BARC. The majority of Rh D-positive individuals with allo-anti-D encountered by Jones and colleagues (1995) were DVI. Severe HDN has been reported in Rh D-positive babies born to DVI mothers with anti-D (Lacey et al. 1983). DVI can arise from three different genetic backgrounds (see Figure 5.3). The common feature of all three types is the replacement of exons 4 and 5 of RHD with exons 4 and 5 of RHCE. In type II, exon 6 is also replaced by RHCE and in type III exons 3 and 6 are also replaced by RHCE (Wagner et al. 1998). The number of D sites/red cell on DVI type I was found to be 500, 2400 on type II and 12 000 on type III (Wagner et al. 1998). Most monoclonal anti-D do not react with DVI red cells, and DVI red cells react with only about 35% of anti-D made by D-negative subjects (Lomas et al. 1989). From this it can be deduced that the amino acid sequence encoded by exons 4 and 5 of the D polypeptide is the most immunogenic region of the D polypeptide (Colour Plate 5.3).
Monoclonal anti-D reactive with DVI red cells should not be used to D type patients because of the risk that a DVI patient would then be typed as D positive and might be transfused with D-positive blood.
Sixty-eight out of 60 000 German blood donors had the D variant DVII (Flegel et al. 1996). This variant results from a Leu110Pro substitution in the D polypeptide (Rouillac et al. 1995). DVII is characterized serologically by its reaction with anti-Tar (Lomas et al. 1986).
DNB is a D variant with a frequency of up to 1 in 292 in white people. Anti-D is found in individuals with the DNB phenotype, which results from a Gly355Ser (predicted to be in extracellular loop 6) substitution in the D polypeptide. No adverse consequences as a result of pregnancy or transfusion have been attributed to the DNB phenotype. Almost all monoclonal anti-D used for routine blood typing would be reactive with DNB cells, so current serological practice would not avoid exposure of DNB-positive individuals to D-positive blood if transfusion were required (Wagner et al. 2002a). An analogous D variant, DWI, was described in an Austrian patient with allo-anti-D. In this case the amino acid substitution Met358Thr was found (Kormoczi et al. 2004).
Most white people with D variants described as weak D have weak D type 1 (Val270Gly), type 2 (Gly385Ala) or type 3 (Ser3Cys; Cowley et al. 2000; Wagner et al. 2000a). Production of anti-D in a D-negative patient transfused with weak D type 2 red cells (450 D antigen sites/cell) has been recorded (Flegel et al. 2000). Anti-D alloimmunization by weak D type 1 red cells has also been reported (Mota et al. 2005).
Common D Variants in Black People
D variants appear to be more common in black people than in white people or Asians; 11% of anti-D in pregnancies in the Cape Town area, South Africa, occurred in D-positive women (du Toit et al. 1989). The D variants found in black people fall into three clusters known as the DIVa, DAU and weak D type 4 clusters (Wagner et al. 2002b).
DIVa is defined by the presence of the low-frequency antigen Goa, an antigen found in 2% of black people (Lovett and Crawford 1967). Anti-Goa has caused HDN. DIVa differs from D at three amino acids (Leu62Phe, Asn152Thr and Asp350His; Rouillac et al. 1995). The DIVa cluster is characterized by Asn152Thr and also includes DIII type 4 and Ccdes.
Five DAU alleles are recognized (DAU-0 occurs in white people and Asians). All DAU types share a Thr379Met substitution predicted to be within the twelfth transmembrane domain. In addition, the amino acid substitutions distinguishing DAU 1–4 are Ser230Ile in DAU-1 and Glu233Gln in DAU-4, both predicted to be located in exofacial loop 4. The substitutions Arg70Gln and Ser333Asn in DAU-2 and the Val279Met substitution of DAU-3 are predicted to be located in intramembranous regions. Anti-D immunization was recorded in DAU-3. DAU-1, DAU-2 and DAU-4 were not agglutinated by most commercial monoclonal IgM anti-D and so patients would be typed as D negative and receive D-negative transfusions. DAU-1 cells had 2113 antigen sites per cell, DAU-2 cells 373 sites per cell, DAU-3 cells 10 879 sites per cell and DAU 41 909 antigen sites per cell (Wagner et al. 2002b).
The weak D type 4 cluster is characterized by Phe223Val in the D polypeptide and includes DOL and many alleles sharing Phe223Val and Thr201Arg. DAR is a partial D variant functionally the same as weak D type 4.2. Five out of 326 black South Africans (1.5%) had the DAR phenotype. DAR differs from D at three amino acids (Thr201Arg, Phe223Val and Ile342Thr; Colour Plate 5.3). One out of four Dutch African black people with the DAR phenotype produced anti-D after multiple transfusions with D-positive blood (Hemker et al. 1999). The D variant, DIIIa, falls into this cluster (the phenotype results from three amino acid substitutions in the D polypeptide, Asn152Thr, Thr201Arg, Phe223Val). Eight out of 130 patients with sickle cell disease were found to have one of the phenotypes DIIIa, DAR or DIIIa/DAR. Three of these patients (one DAR phenotype and two DIIIa/DAR) had made anti-D (Castilho et al. 2005). Castilho and colleagues suggest that DIIIa and DAR typing should be considered prior to transfusion for sickle cell patients who are likely to require multiple transfusions over a long period. A structural model of DAR polypeptide reveals that the amino acid sequence changes defining this phenotype are not predicted to be accessible at the cell surface (Colour Plate 5.3) and yet red cells of this phenotype stimulate anti-D. This suggests the mutations alter the spatial relationship between surface residues of the D polypeptide to create a structure which is sufficiently different from that of D polypeptide to cause the normal D polypeptide to be recognized as foreign antigen when transfused to DAR positive individuals. Multiple points of contact between Fab and D polypeptide would be consistent with structural models (Colour Plate 5.2) and have been proposed previously to account for the fact that most anti-Ds have similar antigen-binding sequences utilizing restricted immunoglobulin genes of the IGHV3 superfamily (Chang and Siegel 1998; Dohmen et al. 2006). However, it does not follow that such an anti-D made by a DAR+ individual would have the same affinity or clinical significance as that made by individuals of phenotype DVI where a much greater alteration of D polypeptide structure occurs (compare plate, and see discussion above).
Common D Variants in Asians
The commonest D variant found in Asian populations is Del (see previous section for discussion of this phenotype). The Del phenotype in Asians is characterized by a silent mutation in exon 9 of RHD (G1227A). G1227A apparently alters RNA splicing with the result that transcripts are generated with exon 9 spliced out (Zhou et al. 2005). It seems likely that this splicing abnormality causes the grossly reduced levels of normal D polypeptide at the red cell surface that are characteristic of this phenotype. Chang et al. (1998) reported deletion of 1013 bp encompassing exon 9 of RHD as a cause of Del, but others consistently report G1227A as the causative mutation (Chen et al. 2004; Yan et al. 2007) and the validity of this study has been questioned (Kormoczi et al. 2005). Yasuda et al. (2005) report a case of anti-D presumed to be stimulated by Del blood resulting from G1227A in a 67-year-old Japanese woman who had previously received D+ blood without making detectable anti-D. There was no evidence of haemolysis upon transfusion of Del blood. Kormoczi et al. (2005) cite one case of a Japanese Del+ woman who developed anti-D during pregnancy and gave birth to two D+ children who presented with no signs of haemolysis. It appears there is little evidence to suggest transfusion of Del blood to a D negative recipient causes clinical problems.
Weak and Partial Antigens Encoded by RHCE
There is evidence of the existence of several variants of E. Of 58 250 Japanese samples that reacted with polyclonal anti-E, eight failed to react with a monoclonal anti-E; three out of these eight that were tested with anti-EW were all negative, indicating that the new variant was different from EW. None of the eight had anti-E in their serum. Most, but not all, anti-E IgM monoclonals reacted with E variant cells; all but one reacted with papain-treated cells. This aberrant expression of E was shown to be inherited; the variant was shown to be different from another described by Lubenko and colleagues (1991) (Okubo et al. 1994). Sera recognizing other variants such as ET are no longer available (Daniels 2002). The genetic bases of four patterns (EI–EIV) of reactivity observed with a panel of monoclonal anti-Es were determined by Noizat-Pirenne and colleagues (1998). EI (Met167Lys) correlates with expression of Ew (Strobel et al. 2004). EII results from a hybrid RHcE-D(2-3)-cE allele, EIII from two amino acid substitutions (Gln233Glu , Met238Val) and EIV from a single amino acid substitution (Arg201Thr). The molecular bases of three E variants found in Japanese are described by Kashiwase and colleagues (2001). A search for RHCE variants in German blood donors with weak expression of Cc and/or Ee antigens revealed the presence of numerous different mutations causing amino acid substitutions in the resultant polypeptide (Doscher et al. 2009; Bugert et al. 2009). Most of the mutations altered the amino acid sequence of the polypeptide in regions predicted to be in transmembrane regions – a pattern similar to that seen with weak D samples (Figure 5.4). CE variants are not usually associated with clinical problems related to transfusion.
Antigens of the Rh System Other than C, c, D, E and e
G
Almost all red cells that carry D and all cells that carry C also carry an antigen G (Allen and Tippett 1958). Amongst the findings that this observation helps to explain is that about 30% of D-negative subjects who are deliberately immunized with Dccee red cells make an antibody that reacts with D-negative, C-positive red cells, the explanation being that the donor cells elicit the formation of anti-G which, as implied above, reacts with all C-positive red cells. The G antigen seems to be defined by Ser103, which is common to both the D and the CE polypeptide when C is expressed (Faas et al. 1996).
Very rarely, a sample may be D positive but G negative (Stout et al. 1963), or C and D negative but G positive, when it is called rG (Race and Sanger 1975, p. 202). The number of G sites on red cells of various Rh phenotypes was estimated, using an eluate made from G-positive cE/ce cells previously incubated with 125I-labelled IgG anti-DC. Results were as follows: DCe/DcE, 9900–12 200; dCe/dCe, 8200–9700; and DcE/DcE 3600–5800 (Skov 1976). If rGr cells are not available, anti-G can be made by eluting anti-DC from dCe/dce cells and then re-eluting from Dce/dce cells. However, not all non-hyperimmune anti-DC sera contain anti-G (Issitt and Tessel 1981).
Cw, Cx and MAR can be regarded as forming an allelic subsystem. Cw and Cx are low-frequency antigens that behave as if they are antithetical to a high frequency antigen, MAR (Sistonen et al. 1994). The CE polypeptide amino acid substitutions Gln41Arg and Ala36Thr define Cw and Cx respectively (Mouro et al. 1995). The frequency of Cw in most white populations is less than 2% and that of Cx less than 1%, although both are substantially commoner in Finns. Anti-Cw has caused HTR and HDN and anti-Cx, HDN. The only example of anti-Mar described so far did not cause HDN.
‘Joint Products’ of RHCE
Ce is a product of C and e in cis. Most anti-C sera contain separable anti-Ce (or -rhi), which reacts with cells from subjects of the genotype DcE/Ce but not with those of DCE/ce (Issitt and Tessel 1981). A simple explanation for the high frequency of anti-Ce is offered by structural models of the CE polypeptide, which suggests that the amino acids defining C and e specificity are in close proximity (Colour Plate 5.2). Anti-Ce has been the cause of HDN requiring exchange transfusion (Malde et al. 2000; Wagner et al. 2000b; Ranasinghe et al. 2003). An IgA autoantibody with anti-Ce specificity has been the cause of autoimmune haemolytic anaemia (Lee and Knight 2000).
ce or f
When c and e are in cis, they determine a compound antigen ce(f); for example, ce is determined by DCE/dce but not by DCe/DcE and can distinguish between these two genotypes. Anti-ce is a common component of anti-c and anti-e sera and has been implicated as the cause of HDN (Spielmann et al. 1974) and delayed haemolytic transfusion reaction (O’Reilly et al. 1985).
CE and cE
Antibodies to these compound antigens have also been found though much less frequently than antibodies of specificity anti-Ce and anti-ce (see Race and Sanger 1975).
V and VS
V(ces) is an antigen found in about 27% of black people in New York and 40% of West Africans but only very rarely in white people. VS and V typing of 100 black South African blood donors revealed 34 of phenotype VS+V+, 9 VS+V− and 4 VS−V+ with weak V (Daniels et al. 1998). These authors concluded that anti-VS and anti-V recognize conformational changes in the Rh polypeptide resulting from a Leu245Val substitution and that anti-V was also affected by an additional substitution (Gly336Cys). Clinically significant anti-V and anti-VS have not been reported.
Other Rh Antigens
These are listed in Table 5.1. As already mentioned, some 20 Rh antigens have a frequency in white people of less than 1%; most of these low-frequency antigens are associated with altered expression of the main Rh antigens (see Daniels 2002).
The low-frequency antigen HOFM, associated with depressed C, has not yet been proven to be part of Rh (Daniels et al. 2004). Another rare antigen, Ola, associated with weakened expression of C or E or both, is determined by RHAG (Kornstad 1986; Tilley et al. 2010).
Red Cells Lacking Some Expected Rh Antigens
D− − is a very rare phenotype in which there is no expression of C, c, E or e. In subjects who are homozygous for the relevant allele, the red cells appear to have an abnormally large amount of D antigen, as judged by their agglutination in a saline medium by most sera containing incomplete anti-D. As mentioned above, the red cells have an increased number of D sites. With one sample, the amount of lysis produced by the complement-binding anti-D serum ‘Ripley’ (Waller and Lawler 1962) was found to be 50–70% compared with not more than 5% for cells of common Rh phenotypes (Polley 1964).
D• • is another very rare phenotype in which D is expressed without C, c, E or e. The red cells, unlike those of the phenotype D− −, carry a low-incidence antigen, ‘Evans’ (Contreras et al. 1978). Red cells that are homozygous for the relevant allele have more D sites than DcE/DcE cells but less than those of subjects who are homozygous for the allele determining D− −.
Dc− is a haplotype that determines increased D, decreased c and some f (Tate et al. 1960). Not all Dc− haplotypes express f (Race and Sanger 1975). Two individuals homozygous for DCw− have been reported. This phenotype expresses elevated D antigen and depressed Cw but lacks C and c antigens (Tippett et al. 1962; Huang 1996).
Several examples of D− −, D• •, Dc− and DCw− have been analyzed at the DNA level. It has been reported generally, although not exclusively, that the phenotype results from a normal RHD in tandem with an altered RHCE, in which several CE exons are substituted by exons from D (reviewed by Daniels 2002).
Rhnull
A sample of blood that completely failed to react with all Rh antibodies was described by Vos and colleagues (1961) and given the name Rhnull by R Ceppellini (cited by Levine et al. 1964). A second example was described by Levine and colleagues (1964); in this case, the parents and one offspring had normal Rh phenotypes, although the Rh antigens had diminished reactivity; the authors suggested that the Rhnull phenotype was due to the operation of a suppressor gene (XOr) in double dose, and that the relatives with diminished Rh reactivity were heterozygous for the suppressor gene.
A second type of Rhnull phenotype, apparently due to an amorphic Rh haplotype (in double dose) was described later (Ishimori and Hasekura 1967). This kind is referred to as the amorph type of Rh-null to distinguish it from the ‘regulator’ type described above (Race and Sanger 1975, p. 220). Most examples of Rhnull described are of the ‘regulator’ type.
Rhnull cells lack not only Rh polypeptides (D and CE) but are also deficient in the Rh-associated glycoprotein (RHAG), glycophorin B, CD47 and LW glycoprotein. In addition to lacking Rh antigens, Rhnull cells lack LW and Fy5, and have a marked depression of U and Duclos and, to a lesser extent, of Ss. Glycophorin B levels are approximately 30% of normal (Dahr et al. 1987). Rhnull cells of the ‘regulator’ type have defects in the gene encoding RHAG. When RHAG is not expressed normally, the Rh polypeptides are not transported to the red cell surface and so the red cells have the Rhnull phenotype (Chérif-Zahar et al. 1996). Some mutations in RHAG result in low-level expression of Rh polypeptides and give rise to the Rhmod phenotype. Rhmod cells have very greatly weakened Rh antigens and, like Rhnull cells, have a reduced lifespan (Chown et al. 1972) and bind anti-U, -S and -s only weakly.
Individuals with Rhnull of the amorph type lack RHD and have inactivating mutations in RHCE (reviewed in Daniels 2002).
Rhnull red cells exhibit spherocytosis and stomatocytosis and have a diminished lifespan, associated with a mild haemolytic state (Schmidt and Vos 1967; Sturgeon 1970). The red cells have an increased content of HbF and react more strongly with anti-i; the cells also have an increased osmotic fragility and an increased Na+–K+ pump activity (Lauf and Joiner 1976).
In Rhnull subjects the commonest antibody formed in response to transfusion or pregnancy reacts with all cells except Rhnull and is called anti-Rh29.
Transient Weakening of Rh Antigens in Autoimmune Haemolytic Anaemia
This has been observed in an infant; when recovery occurred and the direct antiglobulin test became negative, the antigens became normally reactive (Issitt et al. 1983).
Absence of D from Tissues other than Red Cells
D has not been demonstrated in secretions or in any tissues other than red cells (for references, see seventh edition, p. 343; see also Dunstan et al. 1984; Dunstan 1986). Crossreactivity of some monoclonal anti-D with vimentin in tissues is mentioned in Chapter 3.
RhAG expression appears very early during erythropoiesis and before the appearance of Rh polypeptides (Southcott et al. 1999).
Rh Proteins and Disease
Early studies seeking to find links between RhD expression and disease did not yield any convincing associations (reviewed in Mourant et al. 1978). Busquets et al. (2007) report an increased incidence of biliary complications in recipients of liver transplants mismatched for RhD (30% of 76 cases versus 17% of 269 cases where graft and host were RhD identical). RhD is not expressed in liver so the mechanism for such an association is not obvious. The study was conducted in Barcelona. The frequency of the D negative phenotype is very high in the Basque region and the frequency of many histocompatibility genes is also characteristic/unique to the region (for example KIR genes, Santin et al. 2006) suggesting the possibility that genes other than RHD may influence these findings.
Flegr et al. (2009) report that military conscripts in the Czech Republic who are RhD negative and infected with Toxoplasma (11 of 181; 6.08%) have slower reaction times and consequently have more road traffic accidents than RhD positive Toxoplasma infected conscripts (17 of 709; 2.4%). A larger study will be needed to prove the validity of this association.
Rh-Associated Proteins
Rh-Associated Glycoprotein (RhAG)
When Rh polypeptides (molecular weight approximately 30 kDa) are precipitated by Rh antibodies, ABH-active glycoprotein (denoted Rh-associated glycoprotein or RhAG) is co-precipitated (Moore and Green 1987). A cDNA encoding RhAG was isolated and sequenced and found to encode a protein of 409 amino acids with 12 predicted transmembrane domains and cytoplasmic amino and carboxyl termini. The protein has one extracellular N-glycosylation site on the first predicted extracellular loop, which is the presumed location of ABH antigen activity (Ridgwell et al. 1992). RhAG has a similar overall structure to the D and CcEc polypeptides but is not sequence related. The gene for RhAG is on a different chromosome (6) from that (1) for Rh polypeptides. It is the Rh polypeptides that determine Rh antigen specificity while RhAG is required for the efficient transport of Rh polypeptides to the red cell membrane (Cherif-Zahar et al. 1996). RHAG encodes three blood group antigens (Duclos, RHAG1; Ola, RHAG2 and DSLK (RHAG3). Duclos is a high frequency antigen resulting from a mutation Gln106Glu. Ola is a low frequency antigen resulting from mutation Ser227Leu. DSLK is a high frequency antigen resulting from mutation Lys164Gln (Daniels et al. 2009).
In intact red cells, Rh polypeptides, RHAG, LW glycoprotein, Glycophorin B and CD47 are associated as an Rh membrane complex, which is absent or greatly reduced in Rhnull red cells (see Figure 3.1) (reviewed by van Kim et al. 2006). Analysis of the red cell membranes of an individual with almost complete deficiency of band 3 (band 3, Coimbra – see also Chapter 6) showed absence or gross reduction of the proteins of the Rh complex in addition to deficiency of band 3, glycophorin A and protein 4.2. These results suggest that the Band 3 complex (band 3, Glycophorin A and protein 4.2) is associated with the Rh complex in the red cell membrane (Bruce et al. 2003). Further support for this model is provided from the analysis of patients with hereditary spherocytosis resulting from inactivating mutations in the protein 4.2 gene. These individuals have a gross reduction of CD47 and abnormal glycosylation of RHAG suggesting that interaction occurs between CD47 in the Rh complex and protein 4.2 in the band 3 complex (Bruce et al. 2002). Evidence for a direct interaction between the Rh complex and the red cell skeleton component ankyrin is provided by Nicolas and colleagues (2003). Structural models of RHAG based on the crystal structure of the homologue found in Nitrosomonas europaea have a central pore, which likely explains how the protein functions as a neutral gas channel for CO2, NH3 and possibly O2 and NO (Tanner, 2002; Ripoche et al. 2004 ; Endeward et al. 2008; Burton and Anstee 2008). Heterozygous inheritance of mutations in RHAG leading to substitutions Thr182Gly or Thr 194Cys is described in patients with overhydrated hereditary stomatocytosis. The red cells of these patients are abnormally leaky to monovalent cations (Bruce et al. 2009).
CD47
CD47 (synonym: integrin-associated protein, IAP) contains 305 amino acids, has a heavily N-glycosylated amino-terminal extracellular immunoglobulin superfamily domain and five transmembrane domains with a cytoplasmic carboxyl terminus. It is encoded by a gene on chromosome 3q13.1–q13.2 (Campbell et al. 1992; Lindberg et al. 1994; Mawby et al. 1994). CD47 on murine red cells appears to act as a marker for self as, unlike normal murine red cells, red cells from CD47 ‘knockout’ mice are rapidly cleared from the circulation by macrophages. In the case of normal murine red cells, CD47 on the red cells interacts with the inhibitory signal regulatory protein alpha (SIRPalpha) on macrophages to prevent clearance (Oldenborg et al. 2000). Red cells of mice expressing approximately 50% normal CD47 are cleared from the circulation more rapidly than normal red cells in the presence of red cell autoantibodies (Olsson et al. 2007). CD47 expression on murine red cells decline with age in vivo and upon storage in vitro (Khandelwal et al. 2007; Gilson et al. 2009). Reduced levels of CD47 on stored human red cells has also been reported (Stewart et al. 2005; Sparrow and Healey 2006). Increased adhesiveness of sickle red cells to thrombospondin may be mediated through CD47 (Brittain et al. 2001). Burger et al. (2012) provide evidence that CD47 on aged red cells undergoes a conformational change which allows binding of thrombospondin 1 and phagocytosis of the aged red cells.
Poss and colleagues (1993) describe a murine monoclonal antibody, UMRh, which reacts with a wide range of tissues, such as stem cells, mononuclear cells, granulocytes and platelets, but appears to be different from anti-CD47. UMRh reacts less well with Rhnull and D− − than with cells of common D-positive phenotypes.
LW Glycoprotein (ICAM-4)
As already mentioned, LW glycoprotein appears to be part of the Rh complex; with anti-LW, D-positive red cells react more strongly than D-negative red cells. Nevertheless, LW is a blood group system genetically independent of Rh, LW being on chromosome 19 and Rh on chromosome 1.
The first example of anti-LW was obtained by injecting rhesus monkey red cells into rabbits and guinea pigs (Landsteiner and Wiener 1940, 1941). The resulting antiserum, after partial absorption with certain samples of human red cells (later described as D negative) reacted only weakly with the same cells but reacted strongly with other samples (later described as D positive). Although for a time it appeared that the antibody produced was identical with human anti-D, it was later shown to be directed against a different specificity to which the name LW (Landsteiner/Wiener) was given (Levine et al. 1963).
The first evidence that anti-LW was different from anti-D was the finding that the antibody produced in guinea pigs reacted equally strongly with D-negative and D-positive cord blood red cells (Fisk and Foord 1942). Other evidence soon followed: it was found that the injection of extracts of D-negative red cells into guinea pigs induced the formation of an antibody which, although it was not the same as anti-D, resembled it (Murray and Clark 1952; Levine et al. 1961); this antibody was later identified as anti-LW.
The first two examples of anti-LW (‘anti-D like’) in humans were identified in 1955 (Race and Sanger 1975, p. 228); the antibodies gave the same reactions as the animal sera and were later shown to give negative reactions with Rhnull cells. The cells of one of the antibody makers and her brothers were then found to be negative with the guinea pig anti-LW (Levine et al. 1963). A distinction can easily be made between anti-D and anti-LW with the use of pronase, which, unlike other proteolytic enzymes, destroys LW (Lomas and Tippett 1985).
LW antigens may disappear temporarily from the cells of LW-positive people, who can then transiently make anti-LW. The number of LW sites on D-positive red cells was found to be 4400 and on D-negative cells to be 2835–3620 (Mallinson et al. 1986).
Subdivision of LW
The LW antigen and antibody described above are known as LWa and anti-LWa. An antigen, LWb, antithetical to LWa, is found on the red cells of about 1% of the population in most parts of Europe. Anti-LWb has been found rarely in LW (a+ b−) subjects, and anti-LWab has been found in LW (a− b−) subjects, in some of whom LW antigens have been lost transiently (see later). All LW antibodies react more strongly with D-positive than with D-negative red cells and fail to react with Rhnull cells. Auto-anti-LW is mentioned on p. 179 and in Chapter 7. For the effect of anti-LW on the survival of incompatible red cells, see Chapter 10.
Structure and Function of LW Glycoprotein
LW encodes a mature protein of 241 amino acids with an amino-terminal extracellular segment comprising two Ig superfamily domains, a single transmembrane domain and a short cytoplasmic domain (Plate 3.2, Bailly et al. 1994). The LW glycoprotein shows considerable sequence homology with the family of intercellular adhesion molecules (ICAMs) and has also been denoted ICAM-4. The protein is a ligand for several different integrins including LFA-1 Mac-1 on leucocytes (Bailly et al. 1995), GpIIbIIIa on platelets (Hermand et al. 2003) and VLA-4 and alpha v-type integrins (Spring et al. 2001). These interactions suggest that this red cell protein may play a role in erythropoiesis and in haemostasis (reviewed in Parsons et al. 1999; Toivanen et al. 2008).
Rh Antibodies
In this section, the specificities of Rh antibodies are briefly considered together with some of their serological characteristics; Rh immunization by transfusion and pregnancy is considered in later sections.
Naturally Occurring Rh Antibodies
Anti-D
When the sera of normal D-negative subjects are screened in an AutoAnalyzer, using a low-ionic-strength method, cold-reacting IgG anti-D is found in occasional samples. In one series, the frequency was 2.8% in D-negative pregnant women and 3% in males (Perrault and Högman 1972). In another series, the frequency was substantially lower, namely 0.16% in pregnant women and 0.15% in blood donors; in this series the antibodies were detected in the AutoAnalyzer but identified using a manual polybrene test; cold-reacting anti-D could be demonstrated in cord serum and on the red cells of newborn D-positive infants born to mothers whose serum contained the antibody (Nordhagen and Kornstad 1984).
Of four males with cold-reacting anti-D who were given repeated injections of D-positive red cells, two formed immune anti-D; when 51Cr-labelled D-positive red cells were injected into the two subjects who had failed to form anti-D, a diminished survival time was found in one but a strictly normal survival in the other (Lee et al. 1984).
Rarely, anti-D detectable by the indirect antiglobulin test (IAT) at 37°C is found in previously unimmunized subjects; in two men described by Contreras et al. (1987) the antibodies were mainly IgG in one case and wholly IgG in the other; a small dose of D-positive red cells was destroyed at an accelerated rate in both cases (50–99% destruction in the first 24 h; see Chapter 10). In the same series there was one subject with anti-D detectable at 37°C only with enzyme-treated cells in whom the survival of D-positive cells was normal.
Rh Antibodies other than Anti-D
Anti-E is found not infrequently in patients who have not been transfused or been pregnant. Often the antibody can be detected only by the agglutination of enzyme-treated cells; at one centre, 60 out of 146 examples of anti-E found in pregnant women were of this kind (Harrison 1970). The highest incidence was in primigravidae whose partners were no more frequently E positive than in a random sample of the population. In the whole series, only 60% of partners were E positive, reinforcing the conclusion that most examples of anti-E encountered in pregnant women are naturally occurring.
In sera from more than 200 000 individuals (prospective recipients of transfusion, antenatal patients, etc.), the incidence of anti-E in D-positive subjects was greater than 0.1% (Kissmeyer-Nielsen 1965). Most of the antibodies were very weak, however, and the detection of so many examples was perhaps partly due to the use of papain-treated ddccEE cells; only 20% were reactive by the indirect antiglobulin technique. In another investigation, of 218 examples of anti-E detected in a single year, using papain-treated ccEE cells from a single donor, only 14% gave a positive indirect antiglobulin reaction; 21% of the subjects had never had a previous transfusion or pregnancy (Dybkjaer 1967).
Some examples of naturally occurring anti-E are detectable by the IAT at 37°C. In two such cases, E-positive red cells were destroyed at an accelerated rate, although in another subject in whom anti-E could be detected (at 37°C) only with enzyme-treated cells, the survival of E-positive cells was normal. All of the three examples of anti-E were wholly, or mainly, IgG (Contreras et al. 1987).
Examples of naturally occurring anti-C, -Cw and -Cx have been described (for references, see previous editions of this book). The antibodies have been agglutinins tending to react more strongly at 20°C than at 37°C, and to react more strongly with enzyme-treated red cells; one anti-C was shown to be IgM. Other examples of antibodies within the Rh system that may be naturally occurring are anti-Rh 30 and anti-Rh 32. A very low incidence of cold-reacting Rh antibodies with specificities other than anti-D has been reported (Nordhagen and Kornstad 1984).
Cold-Reacting Auto-Anti-LW
In screening 45 000 blood samples in the AutoAnalyzer using a low-ionic-strength polybrene method, 10 examples of auto-anti-LW were found. The sera reacted as well at 18°C as at lower temperatures but did not react at 31–35°C. The titre, as determined in the AutoAnalyzer in eight of the cases, was 8 or less. Three sera were fractionated by DEAE-cellulose chromatography; two of the antibodies appeared to be solely IgG and one to be partly IgM and partly IgG. The cold anti-LW was found to be less positively charged than the bulk of the IgG, unlike immune IgG anti-LW, which resembled IgG anti-D in being more positively charged (Perrault 1973).
Immune Rh Antibodies
As it has long been a routine practice to transfuse D-negative subjects only with D-negative blood, the formation of anti-D as a consequence of transfusion is now uncommon. When an antibody within the Rh system is formed as a consequence of transfusion, it is quite likely to be of a different specificity, such as anti-c, as c is not normally taken into account when selecting blood for transfusion (unless, of course, the recipient is known to have formed anti-c). By contrast, in women immunized to Rh antigens by pregnancy, anti-D was, until the introduction of immunoprophylaxis in about 1970, easily the commonest antibody to be found. At one US centre, 94% of immune antibodies within the Rh system found in pregnant women were anti-D (Giblett 1964). At an English centre the figure (for 1970) was substantially lower, namely 82% (LAD Tovey, personal communication), possibly because examples of non-immune anti-E were included among the Rh antibodies. At this centre the figure for anti-D, as a percentage of all Rh antibodies found in pregnant women, had fallen to 35% by 1989 (GJ Dovey, personal communication).
Of sera containing anti-D, about 30% will also react with C-positive, D-negative red cells and about 2% will react with E-positive, D-negative red cells (Medical Research Council 1954).
In tests on 50 single donor sera containing anti-DC or anti-DCE, 37 were found to react with rG red cells, at first suggesting that these sera contained anti-G. However, after sequential elutions from Ccee and Dccee cells, only three of the original sera contained potent anti-G and a further 12 contained weak anti-G. The reactions of many of the original sera with rG red cells were presumed to be due to the presence of anti-Cc (Issitt and Tessel, 1981). [Anti-CG is a term used for those anti-C sera that react with rG cells (Issitt 1985).]
In sera from immunized patients who have formed Rh antibodies other than anti-D, the antibodies most commonly found are anti-c and anti-E; anti-c reacts with approximately 80% of random samples from white people and anti-E with approximately 30%. Figures for the prevalence of these antibodies are given in Chapter 3.
Anti-ce is present in most sera containing anti-c and in most sera containing anti-e. Anti-CE is sometimes found with anti-D (Race and Sanger 1968, p. 164) or with anti-C (Dunsford 1962).
Anti-C without anti-D is rare. Even in D-negative subjects, C in the absence of D is poorly immunogenic (see below). In sera containing ‘incomplete’ anti-D, anti-C is not uncommonly present as an agglutinin (IgM); such sera are often used as anti-C reagents in blood grouping. The finding of anti-C in a CWpositive person (Leonard et al. 1976) is very rare indeed. Most anti-C sera are mixtures of anti-C and anti-Ce.
Anti-V and anti-VS (es) react with corresponding antigens found most commonly in black people. ‘Anti-non-D’ (Rh 17) is made by D− −, DCw−, Dc− and D• • subjects (Contreras et al. 1979). ‘Anti-total Rh’ (Rh 29) is made by some Rhnull subjects.
Because of the outstanding importance of anti-D, this antibody has been far more thoroughly studied than any other antibodies of the Rh system and the following sections deal exclusively with it. Immune responses to Rh antigens other than D are discussed later.
Characteristics of Anti-D
Most examples of anti-D are IgG and, in a medium of saline, unless present in high concentration will not agglutinate untreated D-positive red cells but can be detected using a colloid medium, polybrene or enzyme-treated red cells, or by the IAT.
A minority of anti-D sera contain some IgM antibody, almost always accompanied by IgG antibody; provided the IgM antibody is present in sufficient concentration these sera agglutinate red cells suspended in saline. Occasional anti-D sera contain some IgA antibody but, in all examples encountered so far, antibody of this Ig class has occurred as a minor component in a serum containing predominantly IgG antibody.
IgM Anti-D
As mentioned above, sera that contain a sufficient amount of IgM anti-D agglutinate untreated D-positive red cells suspended in saline. In a medium of recalcified plasma, diluted up to 1:32 in saline, the titre of purified IgM anti-D is enhanced four-fold; the titre is also slightly enhanced by using enzyme-treated red cells (Holburn et al. 1971a). Several examples of IgM anti-D detectable only with enzyme-treated red cells have been described.
In the early stages of Rh D immunization, it is common to be able to detect anti-D only by a test with enzyme-treated red cells. The finding gives no indication of the Ig class of the antibody. A positive result with enzyme-treated cells is usually soon followed by a positive IAT due to a reaction with anti-IgG.
The number of IgM molecules that can be taken up by a particular sample of D-positive red cells is considerably smaller than the number of IgG anti-D molecules that can be taken up by the same sample. For instance, a particular sample of DCe/dce red cells would take up about 31 000 IgG molecules per cell, but only 11 500 molecules of IgM anti-D (Holburn et al. 1971a).
IgG Anti-D
Undiluted anti-D serum containing only IgG anti-D not uncommonly agglutinates D-positive red cells suspended in saline and, occasionally, with potent IgG anti-D, agglutination may be observed even when the serum is diluted in saline as much as 1 in 100 (M Contreras, personal observations).
Although, apart from the exceptions just mentioned, IgG anti-D in a medium of saline will not agglutinate untreated D-positive red cells of ‘normal’ phenotype, some examples will agglutinate red cells which are heterozygous for the very rare haplotype D− − and most examples will agglutinate D− −/D− − cells (Race and Sanger 1975, p. 214).
IgG anti-D will agglutinate red cells in a variety of colloid media, for example 20–30% bovine albumin will agglutinate enzyme-treated cells suspended in saline and will sensitize red cells to agglutination by an anti-IgG serum.
IgG Subclasses and Anti-D
IgG Rh antibody molecules are predominantly of the subclasses IgG1 and IgG3 (Natvig and Kunkel 1968), although occasional examples are partly IgG2 or IgG4. In testing the serum of 96 Rh D-immunized male volunteers, IgG1 anti-D was present in all cases with, or without, anti-D of other subclasses: IgG3 anti-D was present in many cases, moderately potent IgG2 in eight cases and moderately potent IgG4 anti-D in three (CP Engelfriet, personal observations,). An example of anti-D that was wholly or mainly IgG2 has been described. The donor had been immunized many years previously and the antibody concentration was only 1 µg/ml (Dugoujon et al. 1989).
In demonstrating the presence of different IgG subclasses amongst anti-D molecules it is important to use red cells with a ‘strong’ D antigen (DDccEE rather than Ddccee or DdCcee) as otherwise minor subclass components may be overlooked (CP Engelfriet, personal observations). It may sometimes be helpful to fractionate sera on DEAE cellulose before testing them. For example, an anti-D found to be partly IgG4 by Frame and colleagues (1970) was tested by Erna van Loghem (personal communication), who found the IgG4 component difficult to demonstrate in whole serum but readily demonstrable in a fraction relatively rich in IgG4.
In women immunized by pregnancy, it is common to find that anti-D is composed predominantly of a single subclass; on the other hand, most subjects who have been hyperimmunized by repeated injections of D positive cells have both IgG1 and IgG3 anti-D (Devey and Voak 1974). The findings in another series were similar, anti-D being composed of more than one IgG subclass more commonly in immunized male volunteers than in women immunized by pregnancy (CP Engelfriet, personal observations).
The different effects produced by IgG1 and IgG3 anti-D in monocyte assays in vitro and in causing red cell destruction in vivo are referred to in Chapters 3, 10 and 12.
Different IgG Subclass Composition of Anti-D in Individual Donors and in Immunoglobulin Preparations from Pooled Donations
After incubating D-positive red cells with anti-D sera, the amounts of IgG1 and IgG3 anti-D bound to the cells can be determined using monoclonal anti-IgG1 and anti-IgG3 in a procedure involving flow cytometry. Using sera from 12 hyperimmunized subjects, the mean amount of IgG3 bound was 16% of the total (Shaw et al. 1988). In another series, an almost identical figure (17%) was obtained, with a range of 0–60% (Gorick and Hughes-Jones 1991). In this second investigation, 17 IgG anti-D preparations for immunoprophylaxis were also tested and, unexpectedly, found to deposit less IgG3 on red cells: the mean was 8% of the total, with a range of 1–18%. It was suggested that certain methods of IgG production might result in preferential loss of IgG3.
Anti-D in Relation to Gm Allotypes
In those subjects who make anti-D and who are heterozygous for G1m(f) and G1m(a) there is a preferential production of anti-D molecules bearing G1m(a) (Litwin 1973). Gm allotypes are described in Chapter 13.
In one reported case, an example of anti-D examined in 1957 contained both G3m(b) and G1m(f) molecules, but in a sample taken from the subject 8 years later the antibody carried only G1m(f) (Natvig 1965).
IgA anti-D can be demonstrated by the antiglobulin test, using a suitably diluted anti-IgA, in some sera that contain at least moderately potent IgG anti-D. Although many sera containing IgA anti-D do not agglutinate D-positive red cells in saline, one example containing IgA anti-D with a titre of 128 agglutinated saline-suspended red cells after centrifugation (PL Mollison, unpublished observations). Fractionation of plasma from this later sample confirmed that the agglutinating activity was present in the IgA but not in the IgM fraction (W Pollack, personal communication).
The production of IgA anti-D seems to be associated with hyperimmunization. In one case, following boosting of an already immunized subject, the titre of IgG anti-D rose first and IgA anti-D became detectable only some months later (Adinolfi et al. 1966). Estimates of the frequency with which IgA anti-D is found in hyperimmunized subjects vary. In one series, of 52 sera with IgG anti-D titres of 1024 or more, 50 gave positive results with one anti-IgA serum and 47 gave positive results with another (J James and MG Davey, personal communication). In another series, of 11 hyperimmunized donors, IgA anti-D was found in six, with IgG anti-D concentrations varying from 29 to 75 µg/ml, but no IgA anti-D could be demonstrated in the remaining five donors, including one with an IgG anti-D concentration of 272 µg/ml. No discrepancies were found between tests made with two different anti-IgA sera (seventh edition, p. 351). In another series of hyperimmunized subjects, IgA anti-D was detected in 14 out of 19 (Morell et al. 1973).
Failure of Anti-D to Activate Complement
The vast majority of anti-D sera do not activate complement. If untreated red cells, or cells treated with a proteolytic enzyme, are incubated with fresh serum containing potent incomplete anti-D, no lysis is observed, even using a sensitive benzidine method (Mollison 1956, p. 217). Similarly, in testing red cells sensitized with anti-D, positive results with anti-complement have scarcely ever been reported; the most fully studied example came from a donor ‘Ripley’: freshly taken serum lysed D-positive red cells (Waller and Lawler 1962); the serum also sensitized D-positive red cells to agglutination by anti-C4 and anti-C3 as well as by anti-IgG (Harboe et al. 1963). D-positive red cells take up twice as much antibody when incubated with ‘Ripley’ as when incubated with a normal anti-D serum (NC Hughes-Jones, personal communication).
A mysterious example of a complement-binding anti-D was described by Ayland and colleagues (1978). The donor had a weakly reacting partial D and the anti-D was therefore of restricted specificity; although the antibody was not potent it sensitized red cells to agglutination by anti-complement as well as by anti-IgG.
Almost all examples of anti-D fail to activate complement. Using 125I-labelled C1q and 131I-labelled IgG anti-D, it has been shown that in fact C1q molecules can bind to anti-D on the red cell surface; the number of C1q molecules bound is relatively low (about 100 per cell) when the number of anti-D molecules per cell is 10 000, but when the number of anti-D molecules per cell rises to 20 000, approximately 600 C1q molecules per cell are bound. In experiments in which D-positive red cells were very heavily coated with anti-D as many as 1600 C1q molecules per cell were bound (Hughes-Jones and Ghosh 1981). Nevertheless, when purified labelled C1 is added to red cells coated with anti-D, C1r and C1s are not activated, as shown by absence of cleavage (NC Hughes-Jones, personal communication).
At first sight it is perplexing that although the number of K antigen molecules per red cell is lower than that of D molecules, some examples of anti-K activate complement. The explanation might be that the K antigen unlike D is at some distance from the lipid bilayer, making it easier for two or more IgG molecules to come into close apposition with access to C1q unobscured by associated components of the glycocalyx like Glycophorins A and B which form part of the band3/Rh complex (Plate 3.1; Plate 6.1).
Quantification of Rh Antibodies
Methods of estimating the concentration of anti-D are described in Chapter 8. The approximate minimum concentrations of anti-D detectable by different techniques are as follows: AutoAnalyzer, 0.01 µg/ml; ‘Spin’ IAT, 0.02 mg/ml; two-stage papain test, 0.01 mg/ml; and manual polybrene test, 0.001 µg/ml.
The maximum concentration of IgG anti-D found in serum is about 1000 µg/ml. The lowest concentration of IgM anti-D detectable in a medium of saline is about 0.03 µg/ml (Holburn et al. 1971a), although in a medium of recalcified plasma a concentration of 0.008 µg/ml can be detected (Holburn et al. 1971a,b).
Affinity Constants of Rh Antibodies
The affinity constant of Rh antibodies are heterogeneous both among examples of antibodies of the same specificity from different donors and within the population of antibody molecules of a particular specificity from a single donor (Hughes-Jones et al. 1963, 1964). In 24 examples of IgG anti-D the constant varied from 2 × 107 to 3 × 109 l/mol, with an average of approximately 2 × 108 l/mol (Hughes-Jones 1967). The affinity constants of some other IgG antibodies were as follows: anti-E, 4 × 108 l/mol; anti-e, 2.5 × 108 l/mol; and anti-c, 3.2–5.6 × 107 l/mol (Hughes-Jones et al. 1971). In anti-D immunoglobulin prepared from pooled plasma, the range as expected was less: 18 out of 25 preparations had constants within the range 2 × 108 to 4 × 108 l/mol (Hughes-Jones and Gardner 1970). The affinity constants of monoclonal IgG anti-D tend to be higher than those of polyclonal antibodies: seven monoclonals had values ranging from 2 × 108 to 2 × 109 (Gorick et al. 1988), compared with an average of 2 × 108 for polyclonals.
In a study of 14 monoclonal anti-D, the affinities of the four IgM antibodies (1–4 × 107/M) were found to be lower than those of the 10 IgG antibodies (2.3–3.0 × 108/M). The difference was correlated with the genetic origin and extent of mutation of the rearranged VH and VL germline genes responsible for the variable regions of the Fab pieces. The rearranged genes of three out of the four IgM antibodies (characteristic of the primary response) were all derived from the same VH and VL germline genes and had undergone relatively few point mutations; the VH of the fourth antibody had undergone only a single point mutation compared with the germline. The increased affinity of the 10 IgG antibodies (characteristic of the secondary response) was achieved by two mechanisms. First, by an increase in the number of point mutations in the same genes used by the IgM antibodies, resulting in a better fit between antibody-combining site and antigen; and second, by a recruitment of other highly mutated VH and VL genes, which also resulted in a binding site with a better fit for the antigen. Recruitment of other genes in this way is known as a repertoire shift. Affinity generally increased with increasing somatic hypermutation (Bye et al. 1992).
An ELISA method for measurement of the affinity of monoclonal anti-D gave affinity constants ranging from 1.3–7.4 × 108/M. Affinity constants for polyconal anti-D in maternal samples, immunized donors and anti-D immunoglobulin used for prophylaxis were within the range 1.5–12 × 108/M. The authors argue that measurement of affinity constants using radioiodinated antibodies underestimates the value obtained because of inactivation of the antibody during radiolabelling (Debbia and Lambin 2004, Debbia et al. 2005).
Gene Usage by Rh Antibodies
Antibodies to D antigen use a restricted set of V (notably IGVH3) and J gene segments (Perera et al. 2000). Using a single chain Fv phage antibody library based on the germline gene segment DP50 and light chain shuffling it was shown that the CDR1 and CDR2 sequences of the DP50-based antibodies were common to both anti-D and anti-E and that specificity was conferred by the VHCDR3 sequences and their correct pairing with an appropriate L chain (Hughes-Jones et al. 1999). The selective use of IGHV3 genes is not restricted to anti-Ds made by hyperimmunized donors and IGHV3 genes are also used in anti-C, Anti-G and anti-e (Dohmen et al. 2006; Dohmen et al. 2008). In another study light chain shuffling was used to prepare six D-specific Fab phages paired with the H chain of an anti-D (43F10). The L chains of the six D-specific 43F10(Fab) clones used five different germline genes from three Vkappa families and three different Jkappa segments. The three F(ab)s most reactive with D had light chains with a Ser-Arg amino acid substitution in CDR1 (St-Amour et al. 2003). A similar Ser to Arg substitution of kappa L chains utilized by anti-D has been observed by others (Chang and Siegel 1998; Meischer et al. 1998). Some monoclonal anti-D recognize a ce polypeptide in which Arg145 is substituted by Thr; Thr154 (Wagner et al. 2003). Other monoclonal anti-Ds recognize a ce polypeptide with Ser122 substituted by Leu (Chen et al. 2006). Thr145 and Leu122 are not found in the D polypeptide. This observation and the frequent occurrence of so-called mimicking anti-Rh in the serum of patients with warm-type autoimmune haemolytic anaemia (Issitt and Anstee 1998, p. 962) may be a reflection of the gross homology between the two polypeptides, which stimulates the formation of antibodies with similar structural properties. Another factor that may be relevant to this phenomenon is the cationic nature of anti-Rh antibodies. Boucher et al. (1997) report that anti-Ds utilize VH genes from the most cationic segments available in the human VH repertoire and that the cationic residues are not clustered in the CDRs. They suggest this property is important in allowing high affinity binding of antibody to the D polypeptide by attraction to negatively charged residues in the environment of the D polypeptide (sialic acid residues on glycophorins A and B and the N-glycan of band 3 for example, Plate 3.1).
Rh D Immunization by Transfusion
The Response to Large Amounts of D-Positive Red Cells
When a relatively large amount of D-positive red cells (200 ml or more) is transfused to D-negative subjects, within 2–5 months anti-D can be detected in the plasma of some 85% of the recipients. In about one-half of those D-negative subjects who fail to make serologically detectable anti-D after a first relatively large transfusion of D-positive red cells, further injections of D-positive red cells fail to elicit the formation of anti-D (see section Responders and non-responders, below).
Evidence that some 85% of D-negative subjects will make serologically detectable anti-D after a single transfusion of D-positive red cells is as follows. In one series, following the transfusion of 500 ml of D-positive blood, 18 out of 22 D-negative subjects developed anti-D within 5 months; none of the remaining four subjects made anti-D within 14 days of a further injection of D-positive red cells (Pollack et al. 1971). However, the red cells of this second injection were labelled with 51Cr, and in two of the four subjects without serologically demonstrable anti-D the T1/2Cr was diminished, to 4.8 and 12.1 days respectively (Bowman 1976). The number of subjects primarily immunized was thus 20 out of 22. In another series in which D-negative subjects received 200 ml of red cells, previously stored in the frozen state, 24 out of 28 produced anti-D within 6 months (average time 120 days), and two of the remaining four produced anti-D after a further injection of D-positive red cells (Urbaniak and Robertson 1981). The overall incidence of primary Rh D immunization following an injection of about 200 ml of D-positive red cells in these two series seems therefore to have been over 90% (46 out of 50).
In a follow-up of D-negative patients who had received an average of 19.4 units of D-positive blood during open heart surgery, anti-D was detected in 19 out of 20 cases (Cook and Rush 1974), but this report is made a little less impressive by the fact that in seven of the subjects the antibody was detected only in tests with enzyme-treated cells and in two of these seven the antibody was detectable only on a single occasion and could not be detected subsequently. In a study of 78 D-negative patients who received D-positive blood, anti-D was detected in only 16 patients. The patients belonged to the following diagnostic categories: abdominal surgery, including gynaecological and urological interventions (42%); cardiosurgery (33%); trauma (14%); disseminated intravascular coagulation (5%); and miscellaneous (6%). Most patients received a single-unit transfusion (Frohn et al. 2003). A study of 98 primarily non-oncology D negative patients transfused with D positive blood revealed 22 patients alloimmunized and making anti-D (Yazer and Triulzi 2007). These authors conclude that the probability of making anti-D in response to a D-positive transfusion is much lower in patients than in healthy volunteers.
None of eight D-negative AIDS patients receiving 2–11 units of D-positive red cells developed anti-D; in contrast, all of six D-negative patients with other diagnoses receiving 1–9 units of D-positive red cells developed anti-D within 7–19 weeks of transfusion (Boctor et al. 2003). These observations may relate to the immunosuppression occurring in AIDS patients.
The Response to Small Amounts of D-Positive Red Cells
Following a single injection of 0.5–1.0 ml of D-positive red cells, anti-D has been detected in less than 50% of the recipients in many series; see Table 5.4. The table also shows that if a second injection of D-positive red cells is given at 6 months to the subjects without detectable anti-D, some of them form readily detectable antibody within a few weeks, indicating that the original injection of D-positive cells evoked primary immunization.
Table 5.4 Formation of anti-D after injections of 1 ml of red cells of different Rh genotypes.
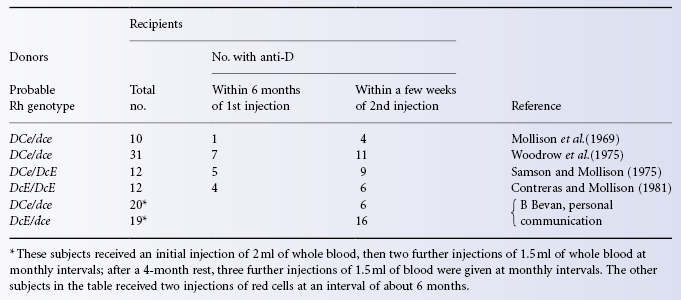
Following an injection of D-positive red cells or a pregnancy with a D-positive fetus, a D-negative subject can be primarily immunized to D without having detectable anti-D in the plasma. For example, in some D-negative subjects injected with 2–4 ml of D-positive red cells, a second injection of D-positive cells given after 6 weeks was rapidly cleared and in these subjects anti-D became detectable later (Krevans et al. 1964; Woodrow et al. 1969). Similarly in 13 D-negative subjects who were injected with 1 ml of D-positive red cells and tested at 6 months, only two had detectable anti-D but five more showed accelerated clearance of a small dose of D-positive red cells and four of these formed serologically detectable anti-D within the following month (Mollison et al. 1969; see Figure 5.5).
Figure 5.5 Survival of 51Cr-labelled D-positive red cells in 11 D-negative subjects, each of whom had received an injection of 1 ml of D-positive red cells 6 months previously. In five subjects (d), survival was below normal from 7–10 days onwards; all of these subjects formed anti-D. Two other subjects (not shown) developed serologically demonstrable anti-D a few months after their first injection. In the remaining six subjects (x), the survival of D-positive red cells was normal and, despite further injections of D-positive red cells, anti-D was never formed. N, normal survival of 51Cr-labelled red cells.
(Source: Data from Mollison et al. 1969. Reproduced with permission of John Wiley & Sons Ltd.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree