Fig. 6.1
A 43-year-old male patient with multiple endocrine neoplasia syndrome (MEN) type 1. On contrast-enhanced MRI a singular mass in the pancreatic head was observed suspicious for a neuroendocrine tumor (top row, left image, white arrow). 68Ga-DOTATATE PET/CT demonstrated corresponding focal somatostatin receptor overexpression of the mass in the pancreatic head (top row, right image, black arrow) and revealed two more focal DOTATATE accumulations in the pancreatic corpus (middle row, right image, black arrow) and cauda (bottom row, right image, black arrow), consistent with multifocal NET of the pancreas. Note that the focal DOTATATE accumulations in the pancreatic corpus and cauda did not show well-defined correlates on MRI or CT
6.3.4 Hybrid Imaging for Suspected Prostate Carcinoma
Basic assessment of suspected primary prostate carcinoma in CUP includes digital rectal examination, serum prostate-specific antigen (PSA) levels, and prostate ultrasound. For local staging, the examination protocol can be extended by multiparametric pelvic MRI, which has been shown to detect 90 % of intermediate- and high-risk prostate carcinomas and additionally identifies lesions eligible for biopsy [37]. According to data from the Swedish Cancer Registry, prostate carcinoma accounts for only 2 % of all deaths in CUP, despite a high prevalence, particularly in the elderly population [38]. The reason for this finding is probably the relatively high detection rate of prostate carcinoma during the primary workup, since cases in which the primary tumor is successfully identified will not be classified as CUP. MRI accurately assesses local tumor extent and infiltration (T staging) and therefore helps to distinguish between low- and high-risk carcinomas [39] – differentiation between these subtypes has a direct impact on therapy decisions. Prostate ultrasound and multiparametric pelvic MRI are valuable tools for local tumor staging (T and N staging). However, in order to detect occult primary tumors and distant metastases (M staging), as well as an accurate assessment of the disease burden, whole-body imaging must be performed.
State-of-the-art diagnostic workup in prostate carcinoma includes hybrid whole-body imaging using the dedicated, prostate-specific radiotracer 68Ga-prostate-specific membrane antigen (PSMA). 68Ga-PSMA PET/CT detects a significantly higher rate of prostate carcinomas and associated metastases compared to 18F-fluorocholine-PET/CT [40]. A recent trial investigated PET/MRI and PET/CT with 68Ga-PSMA and found 68Ga-PSMA PET/MRI to detect prostate cancer and its metastases more accurately [41]. According to the authors, this finding may be explained by the fact that MRI can be performed using organ-specific sequences and provides a higher soft tissue resolution and contrast compared to CT [41]. However, the study findings are limited as the PET/MRI examination protocol focused on the abdomen and pelvis and only included the chest if suspicious lesions were detected on the prior PET/CT scan. Furthermore, PET/MRI detected more suspicious lesions, but there was no final histopathological correlation to exclude false-positive and false-negative findings which are known to occur in hybrid imaging [42]. Advantages of PET/MRI include the lack of ionizing radiation for the MRI component, which makes it an attractive tool for repeated examinations, particularly in younger patients. On the contrary, a whole-body CT scan can be completed within several minutes, while whole-body MRI examination protocols including organ-specific sequences may last more than 60 min. In addition to that, PET/MRI is a relatively novel method, and its true diagnostic value for regional and whole-body staging remains to be investigated in prospective studies. To date, no studies exist which investigate PET/MRI for whole-body staging in CUP. An important aspect to consider is the limited availability of PET/MRI, which to date is restricted to few, mainly academic centers. To conclude, hybrid imaging with 68Ga-PSMA has a significant impact on clinical decision-making and therefore plays a central role in the management of prostate carcinoma patients, especially in low PSA tumors [43]. Therefore, hybrid 68Ga-PSMA whole-body imaging may be considered in all CUP patients with suspected prostate carcinoma.
6.4 CT-Guided Biopsy
In CUP, CT imaging also plays an important role for the identification of lesions eligible for biopsy. Representative tissue samples are required for histological, cytological, and immunohistochemical workup in order to diagnose malignancy and narrow down the potential origin of the primary tumor [44]. Besides lesions in the periphery, tumor manifestations may be accessible via endoscopy or bronchoscopy. CT fluoroscopy-guided biopsy (Fig. 6.2) can be applied to sample even central tumor manifestations with great precision by obtaining real-time moving images to control the positioning of the biopsy needle. This is, however, dependent on the exact anatomic localization and the local expertise and availability of interventional radiology [7]. A study by Hewitt et al. in 149 female patients with peritoneal carcinosis reported that CT- and ultrasound-guided core needle biopsies for histological and immunohistochemical workup were diagnostic in 93 % of cases. Re-biopsy was necessary in 7 % of cases in this population of mainly gynecological malignancies (81 %). Despite these limitations, the study demonstrated that CT- and ultrasound-guided core needle biopsies are a safe and effective diagnostic tool and, under careful consideration of contraindications, may be preferred over open or laparoscopic biopsy [45].
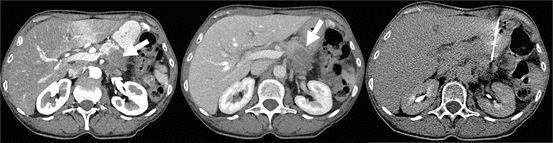

Fig. 6.2
A 57-year-old female patient with an unclear hypodense, hypovascular mass in the pancreatic corpus (white arrows). CT-guided transgastral biopsy (right image) revealed an undifferentiated ductal adenocarcinoma with CK-7 and CK-20 co-expression and weak, partial expression of CDX-2 (Images courtesy of Tobias Geith, MD)
6.5 Magnetic Resonance Imaging (MRI)
MRI allows for staging of malignomas at high soft tissue contrast and spatial resolution [7]. In addition to the depiction of tumor morphology, functional MR imaging techniques are increasingly established for the assessment of various aspects of tumor pathophysiology, including tumor perfusion and diffusivity. Tumor microcirculation can be evaluated using contrast-enhanced or non-contrast-enhanced techniques, with dynamic contrast-enhanced (DCE) imaging as the most widely used method. Diffusion-weighted imaging (DWI) is a noninvasive method reflecting the random Brownian motion of water molecules within the tissue. Although the link between diffusivity and histology is complex, densely cellular tissues exhibit lower diffusion coefficients, and thus diffusion can be useful in tumor characterization. Evidence-based data for the detection of primary tumors using DWI is available for the female breast as well as for the identification of metastasis in whole-body staging [46–48]. Therefore, multiparametric MRI imaging protocols, including morphological and functional sequences, allow for the noninvasive characterization of different aspects of tumor pathophysiology in vivo, with the goal of increased diagnostic accuracy, sensitivity, and specificity.
6.5.1 Whole-Body MRI
Whole-body MRI tumor staging protocols can routinely be performed in less than 1 h examination time, depending on the kind and number of sequences acquired [7, 49, 50], as well as on the acquisition of dedicated sequences for potential characterization and local staging of the primary tumor. In some instances, however, a combination of whole-body staging and dedicated imaging of the primary tumor cannot be achieved, e.g., in breast MRI performed in prone position [7]. Whole-body MRI allows for staging of the local lymph nodes (N staging) as well as distant metastases (M staging). However, studies showed that, compared to 18F-FDG PET/CT, conventional MRI sequences have lower sensitivity and specificity for nodal status assessment. For the detection of lymph node metastases, Antoch et al. observed a sensitivity of 93 % for PET/CT and 79 % for whole-body MRI [46]. This may in part be attributed to the superior spatial resolution of CT compared to MRI. Particularly lymph nodes <12 mm and lymph nodes in anatomic regions prone to pulsation and movement artifacts (hilus lymph nodes of the lung, mediastinum, diaphragm) are frequently not adequately detected in MRI [7]. Additionally, hybrid imaging with PET/CT is able to identify morphologically unsuspicious lymph node metastases by focal tracer accumulation and anatomic co-localization with CT, thereby increasing diagnostic sensitivity (Fig. 6.3). To date, it has not been demonstrated that DWI MRI has the potential to augment diagnostic sensitivity compared to conventional MRI sequences in the identification of lymph node metastases [51]. Improved diagnostic sensitivity of DWI compared to 18F-FDG PET/CT was reported for lung [52] but not for breast cancer [51]. Sensitivity was comparable for the detection of malignant lesions with 94 % for 18F-FDG PET/CT vs. 91 % for whole-body DWI, while specificity was significantly lower for DWI (79 %) than for 18F-FDG PET/CT (99 %). With regard to the identification of distant metastases (M staging), studies report similarly high sensitivity values for DWI and 18F-FDG PET/CT in heterogeneous patient groups with different tumor entities (93–100 %) [46, 53].
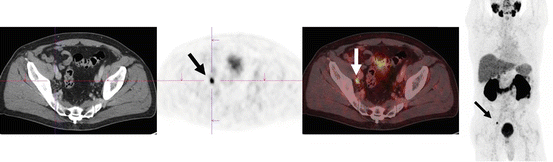

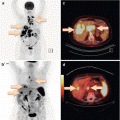
Fig. 6.3
A 67-year-old male with prostate carcinoma following radical prostatectomy 18 months ago and radiotherapy. Increase of prostate-specific antigen (PSA) levels over the last months to 1.5 ng/mL. 68Ga-PSMA PET/CT was performed in search of metastasis and revealed a morphologically unsuspicious lymph node (4 mm) adjacent to the right external iliac artery with focal PSMA uptake (arrows). Histology confirmed a right iliac lymph node metastasis of the prostate carcinoma
Multiparametric MRI, applying different sequences focusing on morphological and functional parameters, is particularly applicable for the detection of metastases in the brain, soft tissue, liver, and muscle. Ultrafast spin-echo sequences, fat-saturated T2-weighted sequences, and contrast-enhanced T1 flash sequences as part of a comprehensive whole-body MRI protocol have been shown to allow for the detection of lung nodules ≤3 mm with similarly high diagnostic sensitivity as compared to multislice CT (MRI sensitivity 87–93 %) [54]. For the detection and characterization of focal liver lesions, multiphasic MRI with contrast-enhanced T1-weighted gradient-echo sequences has been shown to be superior to CT, particularly when imaging with hepatobiliary MRI contrast agents was performed [55].
6.5.2 MRI for Organ-Specific Staging
6.5.2.1 Prostate Carcinoma
Multiparametric MRI of the prostate, including high-resolution morphological sequences, diffusion-weighted sequences, and/or dynamic contrast-enhanced sequences and/or spectroscopy, allows for a multi-facetted characterization of gland anatomy as well as physiological processes. Depending on the imaging protocol, prostate MRI can be performed clinically at 1.5 or 3 T with or without endorectal coil. However, several studies demonstrated that at 3 T, overall accuracy, sensitivity, and specificity for local staging in prostate cancer were not significantly different with or without endorectal coil [56]. In prostate carcinoma, conventional MRI sequences are routinely applied for the detection and precise assessment of tumor size and local extension [7, 57]. Abd-Alazeez et al. investigated the diagnostic value of multiparametric MRI in patients diagnosed with occult prostate carcinoma [58] based on negative ultrasound-guided biopsy and persisting elevated PSA levels. The authors reported a sensitivity of 76 % and a specificity of 42 % for the detection of prostate carcinomas ≥ 4 mm and Gleason score ≥4 + 3 using a multiparametric MRI protocol (T2, DWI, and dynamic contrast-enhanced MRI) at 1.5 T and 3 T with an endorectal coil. In tumors ≥6 mm and Gleason score ≥3 + 4, sensitivity increased to 90 % and specificity remained at 42 %. Multiparametric MRI has been shown to identify focal intraglandular lesions occult on transrectal ultrasound for consecutive biopsy planning. Multiparametric MRI with a T2-weighted sequence and at least two of three functional parameters (DWI, DCE, spectroscopy) has been shown to detect 90 % of prostate carcinomas with intermediate- to high-risk; lower diagnostic performance was demonstrated for very small tumors (<0.5 cm3), tumors in the transitional zone and low-grade carcinomas [37]. Additionally, multiparametric MRI is a strong tool for the precise definition of tumor margins and the reliable assessment of extracapsular tumor extension and potential infiltration of adjacent anatomical structures (Fig. 6.4) [59], such as the seminal vesicles, both important parameters for the correct definition of the T-stadium [7]. Additionally, planning of therapeutic interventions such as radiation or brachytherapy is possible by high-resolution MRI data sets.
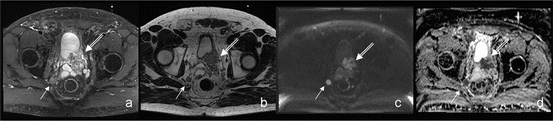

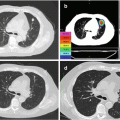
Fig. 6.4
A 68-year-old patient with increased PSA level (6.15 ng/mL). Multiparametric MRI (a) axial contrast-enhanced T1w, (b) axial T2w, (c) DWI b800 with corresponding Apparent Diffusion Coefficient Map, (d) revealed a mass (white open arrow) in left lobe of the prostate infiltrating the bladder and the seminal vesicles, consistent with a T4 prostate carcinoma. Note the suspicious right iliacal lymph node (white closed arrow). Core needle biopsy confirmed a mostly well-differentiated acinar adenocarcinoma of the prostate
6.5.2.2 Breast Cancer
Ipsilateral axillary lymphadenopathy is the first clinical symptom in 0.3–1.0 % of all breast carcinomas [60]. Buchanan et al. investigated female patients with unilateral axillary lymphadenopathy without clinical or mammographic detection of a primary tumor in the breast and reported that contrast-enhanced MRI detected >50 % of these primarily occult breast carcinomas [47]. The authors concluded that in the diagnosis of occult breast carcinomas, MRI is superior to mammography. A study by Orel and colleagues confirmed these results and reported that 86 % of primarily occult breast carcinomas could be detected with MRI [48]. MRI also provides valuable diagnostic information for the local staging of tumors, allowing for a precise definition of tumor margins, evaluation of potential infiltration of adjacent structures such as the pectoral muscle, and the diagnosis of regional lymph node metastases. MR-guided biopsy may be applied to allow for histological sampling of otherwise occult breast carcinoma [7].
6.6 Ultrasound
Depending on the tumor entity, ultrasound can be applied for local and regional tumor staging [7]. Advantages of this fast-evolving imaging modality are low costs compared to cross-sectional imaging modalities, widespread and fast availability, as well as the absence of ionizing radiation. The main disadvantage is the high interobserver variability.
6.6.1 Ultrasound Assessment of Lymph Nodes
Ultrasound with high-frequency probes is well suited for evaluation of superficial lymph nodes. Using morphological criteria, lymph nodes can be classified as malignant, inflammatory, or normal. For the detection of cervical lymph node metastases, Ashraf et al. reported a diagnostic sensitivity of 92 % and a specificity of 97 % for Doppler ultrasound compared to a histopathological standard of reference [61]. In their study, combined ultrasound with Doppler sonography was more sensitive than CT (sensitivity 83 %, specificity 93 %). Mizrachi et al. confirmed these results in a study on cervical lymph node metastases in patients with thyroid carcinoma at a sensitivity of 95 % and a specificity of 90 % [62]. On the contrary, ultrasound showed only limited applicability for the assessment of axillary lymph node metastases in patients with breast carcinoma [63]. Negative ultrasound excludes regional lymph node metastases with high likelihood (negative predictive value 96 %). However, if suspicious lymph nodes are detected, ultrasound frequently underestimates the N status. It remains to be investigated whether contrast-enhanced ultrasound can further improve sonographic lymph node staging [64]. Initial evidence for a potential benefit of contrast-enhanced ultrasound was reported by Rubaltelli and coworkers in a population of 540 patients with malignant melanoma. Compared to fine needle aspiration as the standard of reference, the authors observed a sensitivity of 100 % and a specificity of 99.5 % for contrast-enhanced ultrasound in the detection of lymph node metastases [65].
6.7 Mammography
Women with solitary axillary lymphadenopathy are considered a particular subset of CUP patients, as 75 % will be diagnosed with breast carcinoma. Diagnosis and therapy are following the clinical guidelines for lymph node-positive breast cancer [66]. Less frequent differential diagnoses include lung cancer, amelanotic melanoma, lymphoma, and soft tissue sarcoma as primary tumors [7]. Following the clinical guidelines, diagnostic workup includes clinical history and examination, bilateral mammography in two views, as well as bilateral breast ultrasound. Suspicious lesions should undergo image-guided biopsy with histological and immunohistochemical workup including receptor status and c-erbB2 expression.
Although bilateral mammography is part of the standard workup in women with CUP syndrome, several studies suggested that mammography has only limited sensitivity for the detection of a clinically occult primary breast carcinoma in women with CUP [12, 67, 68]. Nevertheless, the diagnosis of breast carcinoma is of particular relevance, as established therapies are available for breast cancer, resulting in a significantly improved prognosis compared to other, i.e., unfavorable CUP subsets. Therefore, female patients with unremarkable clinical examination, mammography, and ultrasound should undergo contrast-enhanced MRI of the breast to identify otherwise occult carcinomas [69]. Although contrast-enhanced MRI of the breast has a high sensitivity for the diagnosis of breast carcinoma, its low specificity concurrently leads to the recommendation to biopsy suspicious lesions before surgical resection [70].
6.8 Conclusion
Imaging plays a central role in the therapeutic management of patients with CUP. Main challenges include the localization of the primary tumor, the identification of tumor entities with available dedicated therapy strategies, as well as the characterization of clinicopathological CUP subentities. Whole-body imaging stands at the beginning of the diagnostic algorithm in CUP patients, searching for potential primary tumors, identifying all manifestations, and identifying lesions for image-guided biopsy. Due to wide availability and comparably low costs, initial whole-body imaging is frequently performed by contrast-enhanced CT of the neck, chest, and abdomen, although several studies suggest improved diagnostic sensitivity of 18F-FDG PET/CT for the detection of primary tumors and metastatic tumor manifestations [71]. In conclusion, the diagnostic algorithm in CUP patients should primarily aim at patient benefit with the goal to prevent overdiagnosis. Multimodality imaging helps to identify patients with favorable, i.e., treatable CUP subsets, and significantly accelerates the diagnostic workup in CUP [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree