Essential features
Variable features
Acute onset
Perceptual disturbance
Fluctuating course
Hyper/hypoactive
Inattention
Altered sleep/wake pattern
Disorganised thoughts/speech
Emotional disturbance
Fluctuating consciousness
Confusion
Potential examination signs
Autonomic dysfunction
Dysarthria
Tachycardia
Dysnomia
Hypertension
Dysgraphia
Sweating
Aphasia
Flushing
Nystagmus
Dilated pupils
Ataxia
Tremor
Myoclonus
5.2 Causality and Pathophysiology
The pathophysiology of delirium is poorly understood. Proposed mechanisms have included deficiency of acetylcholine, dopamine excess and other neurotransmitter changes, inflammatory processes, metabolic derangement, electrolyte disorders and genetic factors [12]. However, the hypothesis that uncompensated central anticholinergic activity can precipitate delirium is considered the most important. Raised levels of serum anticholinergic activity have been demonstrated in patients with postoperative delirium and are thought to be precipitated in response to acute physiological stress, fever, infection or medication. Patients, who are unable to compensate for the raise in anticholinergic activity in the brain, for example, those with underlying cognitive impairment or dementia, develop the clinical signs of delirium [13]. Reduction in serum anticholinergic activity has been demonstrated with resolving delirium symptoms. Neuroinflammation is also implicated in delirium, with elevated levels of interleukin-1B, and consequently, cortisol, found in the CSF of patient’s post-surgery for hip fracture [14]. The inflammatory cascade is thought to disrupt the blood brain barrier, causing cytokine activation and neurotransmitter deregulation. This type of CNS insult may explain why not all episodes of delirium are fully reversible [15].
Delirium in the older person is often multifactorial (see Tables 5.2 and 5.3). A person is at risk when underlying cognitive impairment or dementia is present, or with increasing age, functional dependence, multiple comorbidities or multiple medications. When admitted to a hospital, patients at risk of delirium should be identified and multicomponent prevention strategies should be implemented (see Table 5.4). These strategies should focus particularly on hydration (oral, intravenous or subcutaneous fluids and assisted feeding programmes if necessary), correction of sensory impairment (visual aids, portable amplifying devices, modified equipment such as large print information booklets), enablement and mobility, maintenance of the sleep/wake cycle (noise reduction, relaxation techniques), cognitive stimulation (communication boards, reorientation, cognitive stimulating activities such as word games or discussion of current events), medication (avoidance and review) and avoidance of unnecessary indwelling medical devices such as urinary catheters and intravenous cannulas [16]. Multicomponent intervention strategies are effective and have been shown to reduce incident delirium in hospitalized patients by 30% [22, 23], with similar results shown in patients offered with proactive comprehensive geriatric assessment [24].
Non-correctable | Correctable | Potentially correctable |
|---|---|---|
Age | Malnutrition | Uraemia. Blood urea >10 |
Male | Dehydration | Depression |
MCI/Dementia | Low albumin | Acute CVA |
Parkinson’s disease | Social isolation | Prolonged hospital stay, >9 days |
Renal and hepatic disease | Sleep deprivation | Severity of acute illness |
History of CVA | Hospital environment | Urinary incontinence |
History of falls and poor mobility | Physical restraint | |
Previous episode of delirium | Indwelling medical devices (IDCs, cannulas) | |
Previous functional dependency | New addition of three or more medications | |
Polypharmacy | ||
Sensory impairment |
1. Medications: polypharmacy, addition of new medication, withdrawal of prescription medication, benzodiazepines, anticholinergics, OTC and herbal medications, substances of abuse |
2. Alcohol intoxication or withdrawal, nicotine withdrawal [25] |
3. Sepsis, systemic illness, hypotension |
4. Hypoxia, hypothermia, hypoglycaemia |
5. Dehydration, anaemia |
6. Electrolyte disturbance (calcium, sodium, phosphate, magnesium) |
7. Nutritional deficiencies (thiamine, B12, folate) |
8. Acute liver or renal failure. Acute cardiac events have not been shown to be associated with delirium [15] |
9. CVA, seizures, vasculitis, encephalitis, meningitis |
10. Pain and analgesia |
11. Constipation, urinary retention |
12. Surgery, especially cardiac and orthopaedic. ICU admission and ventilation |
13. Cancer and terminal illness, brain metastasis |
14. Exposure to the unfamiliar hospital environment and multiple moves around the hospital [26] |
Patient targeted: |
• Correction of sensory impairment |
• Hydration, nutrition |
• Orientation to time, place, person (provide a clock) |
• Monitor, investigate and treat pain, including the use of non-pharmacological pain management |
• Enablement plans to maintain function and mobility |
• Maintain continence with regular toileting, monitor bowels |
• Avoid physical restraint and indwelling medical devices such as urinary catheters and intravenous cannulas |
• Have awareness and respect for cultural and religious sensitivities |
Environmental: |
• Orientation to the hospital environment and reduce the number of room moves around the hospital |
• Provide personal items (i.e. photographs) familiar to the patient |
• Minimize noise |
• Maintain sleep/wake cycle |
Medication: |
• Review medications and de-prescribe if possible |
• Identify high-risk medication (such as benzodiazepines, anti-cholinergics) |
• Monitor for potential medication withdrawal |
Identify and treat reversible medical problems: |
• Dehydration, malnutrition |
• Electrolyte abnormalities |
• Hypoxia, hypotension |
• Renal impairment |
• Urinary retention, constipation |
• Depression, emotional distress |
Education: |
• Education across all staff to promote awareness and early detection of patients at risk |
• Development of local best practice guidelines |
• Identify “champions” to lead implementation of prevention strategies |
5.3 Detection
Despite validated tools to detect delirium and more awareness of the syndrome, 30–67% of delirium in medical inpatients remains undetected, leading to potential complications and prolonged inpatient stays [9, 27]. Gold standard for diagnosis would be with comprehensive geriatric assessment and use of the DSM-V diagnostic criteria for delirium. However, this is time-consuming and not always practical in an acute setting. The Australian and New Zealand Society for Geriatric Medicine [28], the American Geriatrics Society [29] and the British Geriatric Society [30] all recommend the confusion assessment method (CAM, see Table 5.5) as a validated screening tool to detect delirium in elderly patients. The CAM, unlike the MMSE or clock-drawing test, was designed specifically to detect delirium and is user friendly but requires initial training. It has a pooled sensitivity of 82% in medical and post-surgical patients and a specificity of 99% [31]. The original CAM has also been adapted into over ten languages and validated for use in other settings [32], such as the CAM-ICU (for ventilated patients), CAM-ED, nursing home CAM and the family CAM for carers of elderly people living in the community [33]. Interestingly, reasonable sensitivity and specificity in detecting delirium have been obtained through simple screening questions aimed at family and carers. The single question in delirium [34], “Do you think [name] has been more confused recently?” demonstrated a sensitivity of 80% and specificity of 71% in small trials of oncology patients. It has potential as an initial screening tool, particularly in “time poor” environments such as the ED or GP surgery, but should be followed up with further screening and assessment if positive.
Table 5.5
Confusion assessment method
1. Acute and fluctuating course |
• Is there a change in cognition from the baseline? |
• Does this fluctuate during the day? |
2. Inattention |
• Does the patient have difficulty focusing attention? |
• Do they seem distracted? |
• Is concentration poor? |
3. Disorganized thinking |
• Does the patient have disorganized thinking, rambling speech, or are they incoherent? |
4. Altered level of consciousness |
• Is the patient hyperalert? (i.e. wandering, agitated, aggressive) |
• Is the patient hypoalert? (i.e. drowsy, lethargic, stupor, coma) |
5.4 Differential Diagnosis: Delirium, Dementia and Depression
The clinical overlap between delirium, dementia and depression is complex and can present a diagnostic dilemma to the clinician. Forty-two percent of patients referred to specialist psychiatry services with suspected depression actually have delirium [35], with similar percentages of medical inpatients suffering from depression [36]. Like delirium, depression in the elderly is a common syndrome, with reported point prevalence of major depressive disorder over 9%, increasing to 37% when subthreshold or minor depressive symptoms are included [37]. In a similar way to delirium, risk of depression is increased with multiple comorbidities such as Parkinson’s disease, cerebrovascular disease, cognitive impairment and dementia. Conversely, depression in later life doubles the risk of developing dementia [38]. The clinical features of all three overlap considerably, and a careful history must be obtained from family, carers and other medical practitioners to allow accurate diagnosis (see Table 5.6). The range of presenting features for all three conditions can include agitation, depressed mood, cognitive disturbance, anger, euphoria, hallucinations and delusions. In particular hypoactive delirium with psychomotor retardation can be extremely difficult to differentiate from a major depressive disorder. The rate of onset of symptoms and their pattern of fluctuation throughout the day can give a clue to their aetiology, with acute presentations and rapid fluctuations of symptoms more likely to indicate delirium as the primary diagnosis. Disturbances of mood are likely to be more sustained with depression. Once again, sleep disturbance can be a feature of delirium, depression and dementia, but whereas delirium and dementia can cause complete reversal of the sleep/wake cycle, depression tends to precipitate as initial or late-onset insomnia. Characteristics of psychosis also differ between the syndromes. Typical psychosis of delirium features simple delusions, often related to the environment (i.e. belief that nurses are poisoning them or that they are in prison, not hospital), and visual and tactile hallucinations such as insects on the skin. Psychosis in depression is more complex, often with its roots in reality, and featuring themes of guilt and worthlessness. Persistent thoughts of death and self-harm also occur in over 50% of patients with delirium and are not always a defining symptom of depression [35].
Table 5.6
Delirium, dementia and depression
Delirium | Dementia | Depression | |
|---|---|---|---|
Onset | Acute | Insidious | Variable, insidious |
Course | Fluctuating | Progressive | Diurnal variation |
Increased agitation in evenings | |||
(sundowning) | |||
Consciousness | Clouded | Clear | Clear |
Lethargic, stupor, coma | May become clouded in later stages | ||
Attention | Distractibility | Normal | May be poor |
Inattention | |||
Memory | Poor STM | Poor STM | STM usually normal |
Variable cognitive deficits depending on pathology of dementia | |||
Thinking | Disorganised, incoherent | Difficulty with abstract thought | Intact |
May have thoughts of low worth, guilt or | |||
hopelessness | |||
Perception | Misinterpretation | Hallucinations and delusions more | Complex delusion |
Simple hallucination/delusions | common in later stages, or with | Paranoid psychosis | |
Lewy body pathology | |||
Sleep pattern | Reversal of sleep/wake cycle | More common in later stages | Initial or late onset insomnia |
reversal of sleep/wake cycle | |||
Cognitive testing | Distracted | Attempts to comply and find answers | Poor motivation |
Unable to complete MMSE | “I don’t know” | ||
Physical symptoms | May indicate underlying cause | Non-specific | Fatigue, poor appetite, weight loss |
In later stages, fatigue, weight loss, | |||
anorexia |
The clinical overlap between delirium and depression is unsurprising when you consider the pathophysiological pathways involved. Both delirium and depression are linked with alterations is neurotransmitters, abnormal inflammatory responses (as shown by inflammatory markers in the CSF) and abnormal response to acetylcholine activity [38]. In addition, high levels of plasma cortisol, and the failure of dexamethasone to suppress endogenous cortisol production, occur in delirium, depression and severe dementia [39] and may represent a prolonged stress response in these syndromes.
Of course, these conditions do not occur in isolation, and it is quite likely that a majority of patients are suffering from coexistent conditions. Both delirium and depression are potentially reversible, and some patients may benefit from treatment of both disorders. If pharmacotherapy for mood disorder is required, antidepressants with high anticholinergic burden should be avoided, so as to not exacerbate the symptoms of delirium. Although there are case reports in the literature of ECT use in delirium, routine use cannot be recommended due to insufficient evidence. It does, however, remain a treatment option for treatment-resistant depression [40]. Coexistence of delirium and depression has a significant impact on prognosis and care needs, with a fivefold increase in mortality and nursing home placement and a threefold risk of functional decline at 1 month post discharge, when compared to either syndrome in isolation [41].
5.5 Investigation and Non-pharmacological Management
Extra attention should be given to identifying all potential causes of delirium (see Table 5.3), and targeted treatment should be given to any reversible causes. A comprehensive history from family, carers and the general practitioner should be obtained as soon as possible and should include details on the patient’s baseline function and cognition, including any previous formal cognitive testing. A full medication review, aimed at rationalization and de-prescribing, should be performed on all patients. Baseline observations, such as pulse, BP, oximetry, BSL, ECG and urinalysis, should also be performed in all patients, with further investigation targeted to any suspected causes. Routine workup also includes full blood count, electrolytes and renal function, calcium, thyroid function, urine culture, liver function tests and chest X-ray (see Fig. 5.1). A CT brain is strongly indicated where there are focal neurological findings, a history of falls, anticoagulation or signs of meningism. Consideration should be given to a subsequent MRI brain in patients with prolonged delirium (and no obvious precipitant), a history of cancer and suspected cerebral metastasis or focal neurological signs [28]. A lumbar puncture is indicated in patients with headache, signs of meningism or pyrexia of unknown origin. It is worth remembering that older people often do not present with the classical symptoms of meningitis or encephalitis, and acute confusion may be the only presenting symptom. Clinicians should weigh up the indications for lumbar puncture, with the risk and benefit to the patient, bearing in mind that delayed investigation will reduce the likelihood of accurate diagnosis [42]. Routine EEGs are not recommended and have a low accuracy for detecting delirium in the elderly but may be useful in diagnosing suspected seizure disorders causing delirium. In the delirious patient, EEGs show non-specific findings of global slowing, loss of posterior background rhythm and intermittent delta activity, particularly in the frontal region. However, these findings may be useful in differentiating patients with delirium superimposed on dementia and those with dementia alone, where positive EEG findings are not seen [43]. It may also assist in differentiating non-convulsive status epilepticus from catatonic depressive episodes, which may clinically resemble hypoactive delirium [44].
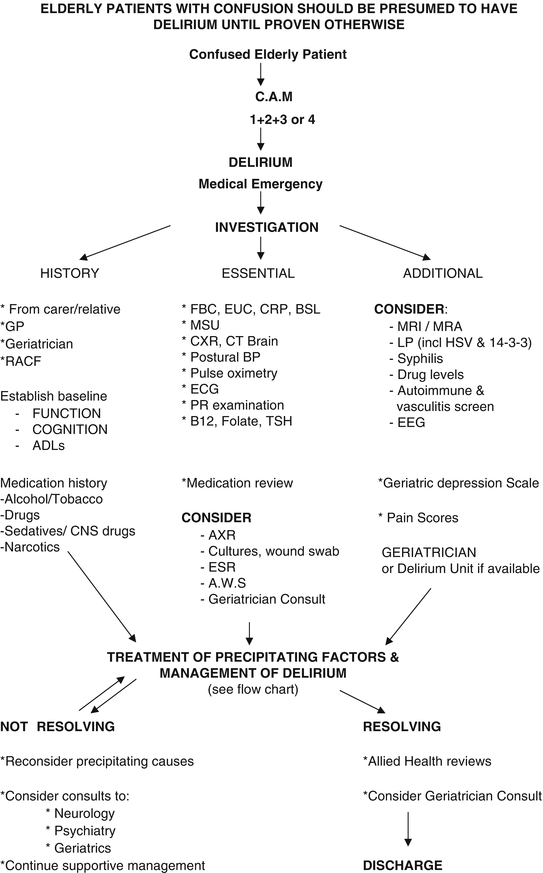

Fig. 5.1
Delirium investigation flow chart
First-line treatment of the symptoms of delirium should be with multicomponent management plans (see Fig. 5.2) along similar lines to prevention strategies. Whereas prevention strategies have shown a reduction in delirium incidence in several clinical trials, once delirium develops, intervention programmes are less effective. Study results have been varied. Some have shown improvement in the severity of delirium symptoms after nurse-led comprehensive delirium programmes [45, 46] and a reduction in falls and trend towards a reduced length of stay [47], but others have failed to show a reduction in hospital mortality, 6-month mortality and admission into residential care [48, 49], nor have they shown an impact on the frequency or recurrence of delirium [20, 21]. Positive outcomes and reductions in mortality have been seen, however, in specialized units such as close observation units [50] (designated areas on general medical wards, with increased nurse-to-patient ratios and comprehensive management programmes), orthogeriatric units and joint medical and mental health units [51]. Despite a need for investment to develop such units, they have been shown to be cost-effective and have significant impact on improving patient experience, carer satisfaction and improving staff attitudes [52].
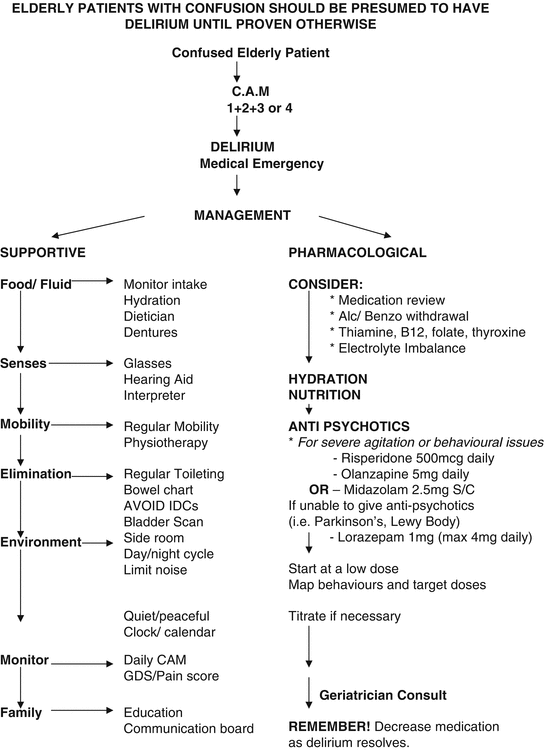

Fig. 5.2
Delirium management flow chart
5.6 Pharmacological Management (See Fig. 5.2)
Pharmacological management of the symptoms of delirium is a controversial but widely adopted practice. Sedative medication, such as benzodiazepines and antipsychotics, are the most commonly prescribed medication and are associated with significant risk to the older person. There are no medications licensed specifically for use in delirium, and none have been shown to reduce the severity, length or recurrence of delirium. Sedative medication should be reserved for patients with severe agitation or aggression, distressing hallucinations or delusions or whose behavioural disturbances pose a risk to themselves or others. Patients with hypoactive delirium should not be prescribed sedative or antipsychotic medication. Traditionally haloperidol has been the agent of choice [53], based on the lack of alternative trial data with second-generation antipsychotics (such as quetiapine, olanzapine or risperidone), rather than a substantial base of evidence supporting its efficacy. Haloperidol has poor sedative properties at low doses, and a previous publication from the Cochrane Database for Systematic Reviews found that higher doses of haloperidol are associated with higher risk of extrapyramidal side effects when compared to olanzapine and risperidone [54] and found there was a lack of robust trial data to support its use. A more recent meta-analysis [55] supported these findings, showing that second-generation antipsychotics are associated with a shorter time to respond and a lower incidence of extrapyramidal side effects when compared to haloperidol. Current clinical guidelines do not recommend the use of haloperidol for either prevention or management of delirium in the older person [56]. What’s more, the use of haloperidol has been associated with a 5% increase in risk of developing delirium in ICU patients [57]. However, second-generation antipsychotics are not without their risks and poor prescribing can lead to over-sedation, falls, urinary incontinence and hospital-acquired pneumonia and is associated with an increased mortality in patients with underlying dementia [58].
Shorter-acting benzodiazepines, such as lorazepam, oxazepam or midazolam, are proven treatments for alcohol withdrawal delirium [59] and may have a role for patients in whom antipsychotic medication is contraindicated (e.g. Parkinson’s disease and Lewy body dementia). Once again utmost caution should be taken when prescribing benzodiazepines as risks include severe sedation, falls, urinary incontinence, hospital-acquired pneumonia and worsening delirium [60]. Despite the theory that there is disruption of cholinergic activity in the brain during delirium, there is no evidence that acetylcholinesterase inhibitors have any role in the treatment of delirium, and their use cannot be supported [61, 62]. Other therapeutics which have failed to show convincing results in small population (<100 participants) clinical trials include melatonin agonists [63] and mood stabilizers [64], and their use cannot be recommended in delirium.
When choosing to use pharmacological treatments for delirium, the following prescribing principles should be considered:
Reserve sedative and antipsychotic medication for patients with severe agitation, aggression or severe behavioural disturbance causing a risk to themselves or others.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



