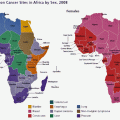
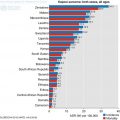
Fig. 8.1
Age standardised incidence and mortality of top 10 cancers in sub-Saharan African women compared to Europe in 2012 (GLOBOCAN 2012)
Within SSA, there is a wide variation in cervical cancer age standardised rate (ASR) of incidence from the highest of 65/100,000 in Mozambique to the lowest of 7.9/100,000 in Sudan and the highest ASR of mortality of 49.8/100,000 in Malawi to the lowest of 5.3/100,000 in Sudan (GLOBOCAN 2012). Similarly, there is a sub-regional variation in the incidence of cervical cancer with the highest ASR incidence and mortality in East Africa and, cervical cancer is less common than breast cancer in southern and western Africa (Fig. 8.2). In West Africa, unlike the other three sub-region, mortality from cervical cancer is less than that from breast cancer (GLOBOCAN 2012).
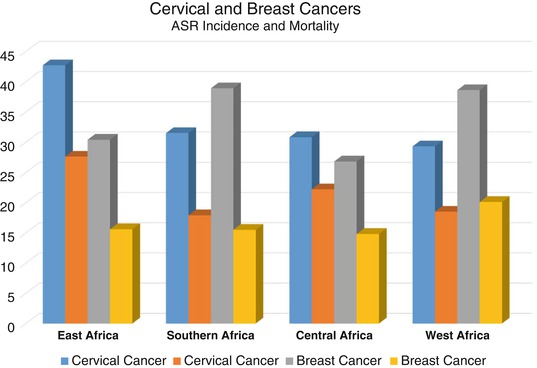

Fig. 8.2
Age standardised rate (ASR) of incidence and mortality per 100,000 of cervical and breast cancers in SSA by sub-regions
There are 500,000 new cases every year worldwide and about 84% of these occur in developing countries (Ferlay et al. 2012). However, new cases of cervical cancer is set to increase by 46.7% in SSA in 2025 from the 2012 level compared to an increase of only 1.7% in Europe (GLOBOCAN 2012, IARC). Most of the increases in SSA will be in women under 65 years of age.
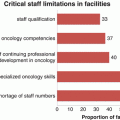
Demographic shifts partially explains the increase in cervical cancer incidence, but other contributing factors include poor preventive measures. Human papilloma virus (HPV) infection is responsible for almost all cases of cervical cancers (Plummer et al. 2016) and despite the proven efficacy of vaccination, worldwide coverage is 1.4% (Lancet Editorial 2016). Across Africa, acceptability of HPV vaccination is high but awareness is low even among healthcare workers (Finocchario-Kessler et al. 2016). Furthermore, screening implementation and utilisation is challenging in Africa because of poor infrastructure, long travel distances, lack of gynaecologists and laboratory pathologists, indadequate record keeping and delayed testing results (Finocchario-Kessler et al. 2016).
In addition to the problems of prevention, late diagnosis is common across Africa with 56–90% of women diagnosed with stage III or IV cervical cancer (Finocchario-Kessler et al. 2016). In a retrospective cohort study with a prospective follow up in North Central Nigeria, Musa et al. (2016) found that 72.3% of cases of invasive cervical cancer were diagnosed at advanced stages (Stage 2B and above) with an overall death rate of 79.8% (Musa et al. 2016). Early diagnosis is crucial and the 5-year survival for stage 1A is 95% but it is 20–30% for stage 4 disease.
8.2 Risk Factors
The risk factors for cervical cancer includes smoking, early age of onset of coitus, multiple sexual partners, early marriage and high parity, HIV/AIDS and marriage to a male whose sexual consorts had cancer of the cervix. It is now apparent that these factors are surrogate markers for genital HPV infection which is by far the most important predisposing factor for cervical cancer.
Genital HPV infection is the commonest sexually transmitted disease, and adolescents are at high risk of contacting the infection. An estimated 80% of sexually active women will be exposed to HPV by the age of 50, but peak exposure occurs in late teens and early twenties. There are more than 40 types of HPV that can infect the genital tract of both men and women. Most of these are symptomless and most infection will regress spontaneously after 6–12 months. There is no treatment that can eradicate the infection. Over time persistent genital infection can lead to cervical cancer and other HPV related diseases including genital warts, vulva intraepithelial neoplasia, vaginal intraepithelial neoplasia and cervical intraepithelial neoplasia (Madeleine et al. 1997; Clifford et al. 2003a, b, 2005; Sotlar et al. 2004).
Of the over 40 genotypes of HPV that affect the genital tract, seven account for 85–90% of cervical cancers worldwide (Munoz et al. 2004), and types 16 & 18 are responsible for about 75% of cervical cancers in Europe (Clifford et al. 2003), while types 6 & 11 account for 90% of genital warts (Von Krogh 2001). In addition to cervical cancer, the oncogenic HPV also induces cancer of the anus, vulva, vagina, penis, mouth and throat. All cases of the approximately 530,000 new cases of cervical cancer annually worldwide, are induced by HPV infection compared to only 25% of vulva cancers, 88% of anal cancers and 31% of oropharyngeal cancers (Plummer et al. 2016).
8.3 Primary Prevention of Cervical Cancer
Genital HPV infection is sexually transmitted the only certain way of preventing infection is by abstaining from all sexual activities or for mutual monogamy in non-infected couples. For those who are sexually active condoms may lower the chance of getting HPV infection if used correctly all the time. However, the recent introduction of HPV vaccine has raised the potential for significant reduction in worldwide incidence of HPV infection and subsequently cervical cancer.
8.3.1 The HPV Vaccine
In a work that was initiated in the mid-1980s, the HPV vaccine was developed in parallel by researchers in Georgetown University Medical Center, University of Rochester, the National Cancer Institute in the USA, and the University of Queensland in Australia. In 2006 the U.S. Food and Drug Administration (FDA) approved the first preventive HPV vaccine, marketed by Merck & Co. under the trade name Gardasil, and by the second quarter of 2007, Gardasil had been approved in 80 countries. Another vaccine called Cervarix, marketed by GlaxoSmithKline, was licensed in Australia in June 2007, and it was approved in the European Union in September 2007. Cervarix was approved for use in the U.S. in October 2009.
The HPV vaccines are subunit vaccines made from major protein of the viral coat or capsid. These virus-like particles mimics the structure of the native virus but do not contain any viral DNA (Syrjänen and Syrjänen 2000). The vaccines elicit virus-neutralising antibody response that prevents initial infection with the HPV types represented in the vaccines.
Cervarix is a bivalent vaccine that protects against HPV type 16 and 18 that accounts for 75% of cervical cancer. Gardasil on the other hand is quadrivalent and protects against HPV type 6, 11, 16 and 18, and therefore in addition to protecting against cervical cancer, it also protects against genital warts. In clinical trials in HPV naïve women both vaccines are over 99% effective at preventing precancerous lesions and subsequently cervical cancer associated with HPV type 16 and 18 (Franco and Harper 2005; Sanofi Pasteur MSD Data on File 06/008). Current studies suggest that protection is maintained for at least 6 years, but based on immune responses it is expected that protection will be extended further. Gardasil is also 99% effective in preventing genital warts associated with HPV type 6 and 11. A summary of efficacy of the two cervical cancer vaccines is shown in Table 8.1 (Herrero and Franceschi 2014).
Study group | Outcome | Quadrivalent vaccine | Bivalent vaccine |
|---|---|---|---|
Young women | Infection efficacy | Proven | Proven |
CIN2 + efficacy | Proven | Proven | |
CIN3 efficacy | Proven | Proven | |
VIN/VaIN 2/3 efficacy | Proven | Provena | |
Genital warts efficacy | Proven | Not a target | |
Anal infection efficacy | Not provenb | Proven | |
Partial cross-protection infection | Proven | Proven | |
Partial cross-protection CIN2+ | Proven | Proven | |
Therapeutic efficacy | None | None | |
Safety | No concerns | No concerns | |
Mid-adult women | Infection efficacy | Proven | Provena |
CIN2 + efficacy | Proven | Not proven | |
Immunogenicity | Proven | Proven | |
Safety | No concerns | No concerns | |
Young men | Infection efficacy | Proven | Not proven |
Genital warts efficacy | Proven | Not a target | |
Anal infection efficacy | Proven | Not proven | |
AIN2 + efficacy | Proven | Not proven | |
Safety | No concerns | No concerns | |
Children | Infection efficacy | Not proven | Not proven |
Disease efficacy | Not proven | Not proven | |
Immunogenicity | Proven | Proven | |
Safety | No concerns | No concerns |
8.3.2 Vaccination Programs
The objective of HPV immunisation programme is to provide three doses of the vaccine to girls before they reach the age when the risk of HPV infection increases and they are subsequently at risk of cervical cancer. Hence in the United Kingdom vaccination is routinely recommended for all girls at 12–13 years of age. However, Cervarix is licensed for individuals from 10 years and Gardasil is licensed from 9 years. There is also evidence suggesting that HPV vaccines are effective in preventing cervical cancer in women up to 45 years of age especially if they had not already been exposed to HPV infection. The benefit-cost is however reduced in older women because of the lower incidence of lesions among women infected by HPV later in life (Herrero and Franceschi 2014) Three dose schedule of intramuscular injections, with flexible dosing intervals if necessary; Cervarix: 0, 1–2 and 6 months and Gardasil: 0, 1 and 4 months. Since the currently available vaccines offer protection against HPV type 16 and 18 that are responsible for only 75% of cervical cancer it is essential to also institute secondary preventive measures even in women who have been vaccinated.
The American Cancer Society (ACS) recommends routine HPV vaccination for girls and boys starting from the age of 11 or 12. It can be started as early as 9 years of age (ACS 2016). It is also recommended for females aged 13–26 years old and males aged 13–21 year old who have not started or completed the series. HPV vaccination is also recommended up to the age of 26 years for homosexual males and for those with weakened immune systems, including people with HIV, if they have not previously been vaccinated (ACS 2016). In an earlier recommendation, members of the Sub-Saharan Africa Cervical Cancer Working Group (2009) did not recommend male vaccination, however the group promised to review the decision when more evidence is available and high vaccination coverage of women had been achieved.
Vaccination programmes for HPV is very variable in sub-Saharan Africa and it is still very low, but with the support of public-private partnerships like the Global Alliance for Vaccines and Immunization (GAVI), some inroads are being made. With the cost of HPV vaccine reduced to US$5 per vaccine dose through the GAVI scheme, Rwanda in its first year under the programme achieved a three-dose vaccination coverage of 93.2% among an estimated 98,762 eligible girls in grade six (Herrero and Franceschi 2014). At this time by January 1, 2012, only Rwanda had a national HPV vaccination programme amongst GAVI eligible countries in SSA (Jit et al. 2014) and only South Africa in non-GAVI eligible countries had a programme (Kim et al. 2013). The Rwandan programme was possible because of initial 3-year donation of two million doses of quadrivalent vaccine Gardasil and 250,000 HPV screening test by Qiagen (Adefuye et al. 2013). After the 3 years, the vaccine was to be offered at a highly discounted price.
8.3.3 Economic Modelling
Using an economic model, Papilloma Rapid Interface for Modelling and Economics (PRIME), Jit et al. (2014) estimated the health and economic benefit of vaccination of girls against HPV before onset of sexual activity in GAVI eligible countries. They estimated that vaccination of 58 million 12-year old girls before the start of sexual activity worldwide will prevent 690,000 cases and 420,000 deaths related to cervical cancer at a cost of US$4 billion. Seventy percent of cancers and 75% of deaths prevented will be in low or lower middle income countries (Jit et al. 2014).
Another economic modelling, which is Excel-based, has projected that vaccination is cost-effective across SSA with 7.9–35.0 cervical cancer cases averted per 1000 vaccinations. Disability Adjusted Life Years (DALYs) projected to be averted, with HPV vaccination coverage of 70% and lifelong protection against HPV 16/18, ranged from 1.28 in Central African Republic to 80,100 in Nigeria (Kim et al. 2013; Table 8.2). HPV vaccine will be cost-effective in most SSA countries at a cost of five US dollars. This is the price currently offered by vaccine manufacturers to the GAVI alliance compared to the price of US$100 the vaccine costs in developed countries (Kim et al. 2013).
Table 8.2
Cervical cancer vaccination model
Country | Cancer incidence (ASR) | Cases averted per 1000 vaccinated | DALYs averteda |
|---|---|---|---|
AFR D | |||
Angola | 30 | 15.19 | 10,220 |
Benin | 35 | 21.83 | 6620 |
Burkina Faso | 28.6 | 15.01 | 9660 |
Cameroon | 24 | 12.12 | 8030 |
Cape Verdeb | 34.9 | 21.66 | 260 |
Chad | 19.9 | 10.12 | 4000 |
Comoros | 51.7 | 31.84 | 650 |
Equatorial Guineab | 25 | 13.49 | 230 |
Gabonb | 24.4 | 15 | 520 |
Ghana | 39.5 | 23.66 | 17,270 |
Guinea | 56.3 | 35.01 | 11,460 |
Guinea-Bissau | 35.1 | 17.49 | 980 |
Liberia | 41.8 | 24.7 | 3170 |
Madagascar | 27.2 | 16.38 | 11,320 |
Mali | 37.7 | 17.63 | 9820 |
Mauritania | 35.1
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||