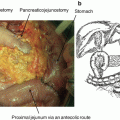
Fig. 18.1
HF10 structure
Gene deletion and insertion of promoters are techniques for modifying viral characteristics in order to increase their selectivity for cancer cells. ONXY-015, a replication-competent oncolytic adenovirus, was used in the first clinical trial for pancreatic cancer [1, 2]. It has been engineered to lack the E1B gene that codes for a 55 kDa protein that binds to tumor suppressor p53 in normal cells and causes progression of the cell cycle and viral replication [3, 4]. E1B-deleted viruses do not generally replicate in normal cells. Since approximately 50–75% of pancreatic tumors lack normal p53, E1B-deleted viruses can replicate in pancreatic cancer cells.
There is another way to enhance replication and oncolysis of tumor cells. A gene that is regulated by tumor-specific promoters is affected in the case of double or triple mutation viruses that complement the gene and activate mutated gene, similar to single-mutation viruses, but only in target tumors. The mutated HSV-1 virus Myb34.5 has a double-mutation locus that comprises both ICP34.5 and ICP6. The β-Myb promoter regulates ICP34.5 (γ34.5) at the ICP6 locus. Thus, the β-Myb promoter complements the deficient γ34.5 gene only in tumor cells [5]. Studies of carcinoembryonic antigen (CEA) [6], Muc-1 [7], ERBB2, amylase, and insulin show that they might be suitable as promoters of an oncolytic virus for the treatment of pancreatic cancer. An upcoming study of a mutated virus using a suitable promoter for pancreatic cancer will show that it resembles the albumin promoter-regulated replication-competent herpes virus against hepatoma 80, or the Muc1 promoter-regulated replication-competent herpes virus against pancreatic cancer [8].
18.3 Dual Mechanism of Action: Local and Systemic Immune Effects
Oncolytic viruses can induce T cell-mediated tumor immunity and cause regression of distant metastases [9–11]. For tumor regression, CD8-positive T cells and NK cells are required because depletion of these cell types has been shown to abolish the antitumor ability of oncolytic viruses. Thus, in addition to their proven efficacy against a variety of tumors through a direct cytotoxic effect, these viruses can activate innate or adaptive tumor immunity, or both [12–15]. In particular, fusion type virus is a strong stimulus for host immunity against tumor antigens. These types of viruses make tumor cell membranes fuse and strongly express tumor cell antigens to antigen-presenting cells. Their effect might be due to changing cell surface characteristics of receptors and ligands, including immune suppressor such as PD-L1.
18.4 HF10 Monotherapy in Eight Patients with Pancreatic Cancer
In 2005, we started a phase I clinical trial of HF10 in patients with pancreatic cancer. The study consists of two stages. The first stage is for three intratumoral injections of HF10, and the second one is for six injections. (Fig. 18.2) The objectives were to evaluate the safety and tolerability in patients with pancreatic cancer. We also examined the tumor response as well as the extent of lymphocyte infiltration induced by viral infection.
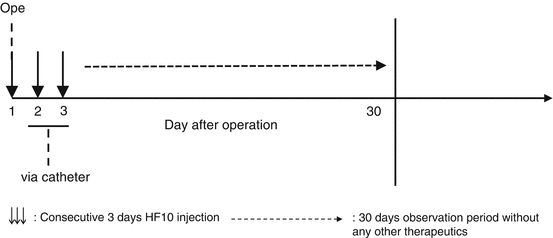

Fig. 18.2
Design of clinical trial for pancreatic cancer. Three down arrows consecutive 3 days HF10 injection, long dashed right arrow 30 days observation period without any other therapeutics
In the first stage, six patients with unresectable pancreatic cancer were enrolled. The first two patients received the intratumoral injections of 1 × 105 pfu on three consecutive days (once intraoperatively and twice postoperatively), followed by the next four patients with the dose escalation up to 1 × 106 pfu. All patients were monitored for adverse effects and efficacy without other additional therapy for 30 days. No treatment-related adverse event was observed in the six patients. The tumor responses were classified as SD in three patients, PR in one patient, and PD in two patients. We also examined the infiltration of CD4 and CD8 cells into tumors with immunostaining. Tumors injected with HF10 had much greater infiltration of CD8 cells than resected tumor specimens that did not receive intraoperative HF10 injection with statistically significant differences. There was more CD4 cells infiltration into treated specimens than in control specimens, but this difference was not statistically significant [16]. The number of NK cells in the peripheral blood increased after injection of virus. Antigen-presenting cells and macrophages were also examined by immunostaining. Macrophages infiltrated the tumor site to a greater extent than in resected specimens that served as control tissue. After only three injections against pancreatic cancer, the CA19-9 levels of Patients 1, 3, and 8 were controlled, and decreased without any other therapy during the 30-day observation period (Fig. 18.3).
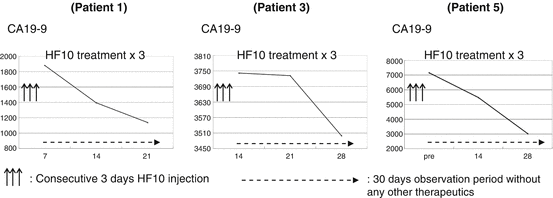

Fig. 18.3
Pancreatic cancer tumor marker movement. Three up arrows consecutive 3 days HF10 injection, long dashed right arrow 30 days observation period without any other therapeutics
During the first stage, we learned that there were some difficulties in the continuous injections through a catheter. Therefore, we developed a new methodology using endoscopy to reach to the pancreatic tumor through the duodenum or gastric wall. It is generally accepted that endoscopic ultrasound (EUS) is useful modality for directly reaching pancreatic tumors with minimal invasiveness. By using the EUS under ultrasound guidance, we were able to directly inject HF10 to the tumor site located in pancreas. In the second stage, two patients were enrolled (Patients 7 and 8). The patients received the three injections by the same route as the first stage + additional three injections via EUS, in a total of six injections. The dose of two patients was 106 pfu/1.0 mL weekly for 3 weeks. The treatment period was thus 4 weeks. None of two showed any treatment-related adverse event. The tumor responses of both patients were PD. Despite the progression, the levels of CA19-9 of the patients were stabilized during the treatment without any other therapy although CA19-9 levels increased again after the treatment (Fig. 18.4).
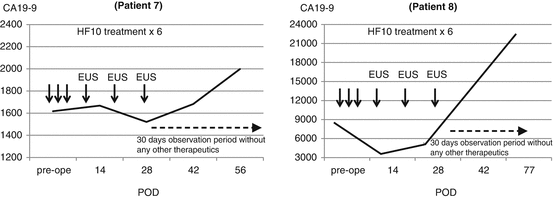

Fig. 18.4
Pancreatic cancer tumor marker movement including EUS method
According to the Choi criteria (modified RECIST criteria), the tumors in Patients 1, 4, 7, and 8 were classified as PD; those in Patients 2, 3, and 6 as SD; and that in Patient 5 as PR. The disease stability (PR + SD) was 50%. In Patient 6, tumor size did not change for more than 80 days after injection therapy, as determined by radiography; this patient did not receive any treatment other than HF10 injection, due to anorexia. These patients survived longer than those who underwent ordinary bypass surgery (cholangiojejunostomy or gastrojejunostomy) for non-resectable pancreatic cancer at our institution in the period, and the median OS was 203 days (6.8 months). In patients with pancreatic cancer who received six injections of HF10, CA19-9 levels immediately increased after the end of treatment although they were suppressed during treatment. We hypothesize that multiple cytokines related to immune response and inflammation promote tumor angiogenesis after treatment. Several oncolytic HSV-1 researchers have reported a relationship between tumor angiogenesis and oncolytic HSV-1 injection [17, 18]. It might be a cause for rapid increases in CA19-9 during the observation period (Table 18.1).
Table 18.1
Non-resectable pancreatic cancer patient profiles
Patient no.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|