Abbreviations
- ACTH Adrenocorticotropic hormone
- ADH Antidiuretic hormone
- AIDS Acquired immunodeficiency syndrome
- AVP Arginine vasopressin
- CSW Cerebral salt wasting
- DDAVP Desamino, d-8 arginine vasopressin
- PAVP Plasma vasopressin concentration
- pOsm Plasma osmolality
- SIADH Syndrome of inappropriate antidiuretic hormone secretion
- V1-3 Vasopressin receptor, types 1-3
Physiology of Hormone Function
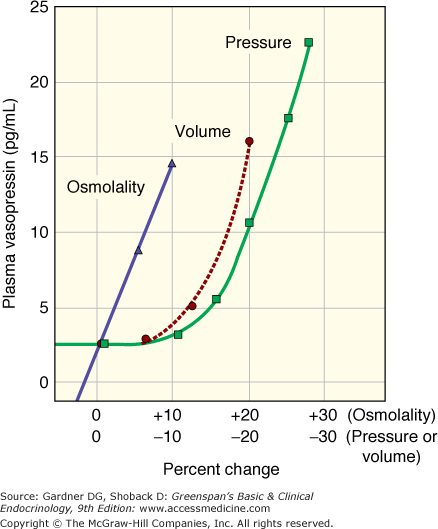
Vasopressin is the water-retaining hormone in all mammals and along with thirst is the primary regulator of osmolality. Pressure and volume are primarily regulated by changes in sodium balance mediated via renin, angiotensin, and aldosterone. The relative importance of vasopressin in regulation of osmolality versus regulation of pressure is reflected in the sensitivity to changes in osmolality versus changes in pressure/volume. Figure 5–1 illustrates the exquisite sensitivity of the osmostat to as little as a 1% change in osmolality. The regulation of vasopressin secretion by baroreceptors, however, involves many concurrent and synergistic sympathetic inputs, and decrease in volume or pressure of 10% to 15% is necessary before there is a measurable increase in plasma vasopressin.
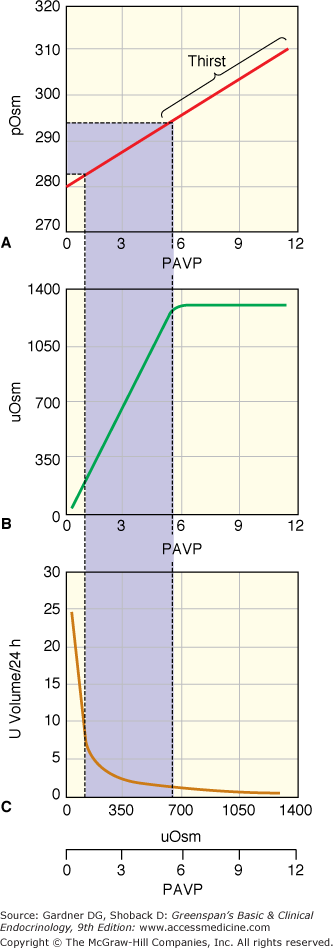
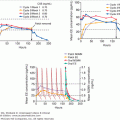
The exquisite sensitivity in the relation of plasma osmolality to urine osmolality and urine volume is illustrated in Figure 5–2. While the normal range of plasma osmolality encompasses a range of approximately 10 mOsm/L, for any individual, the set-point is much more narrow. Small changes in osmolality produce a corresponding and linear change in plasma vasopressin. As illustrated in Figure 5–2, the normal range of plasma osmolality and plasma vasopressin produces a corresponding linear increase in urine osmolality from maximally dilute to maximally concentrated. This entire range from maximally dilute urine to maximally concentrated urine is accomplished by a narrow range of plasma vasopressin (from approximately 1 to 5 pg/mL). In unusual circumstances, plasma osmolality can rise higher than the normal range, and there is a corresponding increase in plasma vasopressin, but urine osmolality plateaus at the concentration of the renal inner medulla. When urine in the collecting duct is iso-osmotic with the urine in the inner medulla, maximum urine concentration is achieved. While the relation of plasma osmolality, plasma vasopressin, and urine osmolality are linear, the relation of these to urine volume is not linear. Rather, there is a logarithmic relationship between urine volume and urine osmolality. The total urine volume required to excrete a fixed quantity of urine osmolytes changes relatively little until plasma vasopressin and urine concentration are nearly absent, then urine volume increases dramatically from a few liters per day to 18 to 20 L/d.
Figure 5–2
Normal physiologic relationship between plasma osmolality (pOsm; mOsm/kg H2O), plasma vasopressin (PAVP; pg/mL), urine osmolality (uOsm; mOsm/kg H2O), and urine volume (L/d). (A) Changes in osmolality induce linear changes in vasopressin with a normal physiologic range of osmolality producing vasopressin levels of 0.5 to 5 pg/mL. (B) The physiologic range of vasopressin produces linear changes in urine osmolality. At vasopressin levels above 5 to 6 pg/mL, urine osmolality is at the maximum, determined by the osmolality of the inner medulla of the kidney. (C) Assuming a constant osmolar load, the relationship of volume to urine osmolality is logarithmic. The urine volume to excrete a given osmolar load at the urine osmolality in (B) is indicated in C. (Calculated from formulas in Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int. 1976;10:25-37.)
(Copyright 2003, A.G. Robinson, University of California at Los Angeles.)
In the kidney, fluid is conserved by reabsorption of sodium and fluid in the distal and the proximal tubules and then reabsorption of water in the collecting duct. The counter-current multiplier system in the loop of Henle generates a high osmolality in the renal medulla. Vasopressin acts on principal cells in the collecting duct by V2 receptors that stimulate the expression of intracellular water channels, aquaporin-2. When vasopressin binds to the V2 receptor, adenylate cyclase is activated to produce cyclic AMP which stimulates both the production of new aquaporin-2 protein and the transfer of existing aquaporin-2 from the cytoplasm into the cell membrane. In the cell membrane, aquaporins act as water channels in the hydrophobic lipid bilayer so that water moves down the osmotic gradient from the collecting duct into the principal cell. Aquaporins-3 and -4 are involved in moving water out of the principal cell into the medullary extracellular fluid and from there into the circulation. In response to changes in vasopressin, aquaporin-2 can be quickly shuttled in and out of the membrane producing rapid changes in water reabsorption and concentration of urine.
Volume/pressure regulation by vasopressin operates through V1 receptors on blood vessels. These receptors cause contraction of smooth muscle to raise blood pressure and, secondarily reduce intravascular volume.
V2 receptors also stimulate antihemophilic and von Willebrand factors. There is a third type receptor (V3) on anterior pituitary cells that stimulates ACTH. These functions are not further considered in this chapter.
In most physiologic situations, changes in osmolality and volume are additive or synergistic in producing the appropriate physiologic response. For example, most cases of dehydration result in greater loss of water than solute. This produces an increase in plasma osmolality and a decrease in volume that act together to stimulate thirst and the secretion of vasopressin, which promotes retention of water. Similarly, excess ingestion of hypotonic fluid produces a decrease in plasma osmolality and an increase in plasma volume, both of which decrease plasma vasopressin and result in excretion of dilute urine. The normal regulation of osmolality is an elegant and simple system. Fluid ingested and water produced from metabolized food is in excess of true need. The retained water causes a small decrease in plasma osmolality with a small decrease in vasopressin and an excretion of the ingested fluid. If the water intake is not sufficient to supply body needs, plasma osmolality rises, producing concentrated urine to reduce fluid loss, and stimulates thirst that induces drinking to replenish body fluid.
Fluid and electrolyte balance is not well regulated in elderly people. By age 80, total body water declines to as low as 50% of the level in normal young adults. Older subjects may have a decrease in glomerular filtration rate, and the collecting duct is less responsive to vasopressin. Numerous studies have indicated a decreased thirst with dehydration in elderly subjects, but also a lessened ability to excrete a water load. These age-related changes in body fluid and renal function predispose elderly people to both hypernatremia and hyponatremia. Also, elderly people are more likely to have comorbid diseases which enhance their tendency to become hyponatremic or hypernatremic. It is incumbent upon the physician to pay special attention to fluid balance in older people.
Anatomy of Hormone Synthesis and Release
The posterior pituitary (unlike the anterior pituitary) is not a gland but only the distal axon terminals of the hypothalamic magnocellular neurons that make up the neurohypophysis. The cell bodies of these neurons are located in paired supraoptic nuclei and paired paraventricular nuclei of the hypothalamus. The paraventricular nuclei are located on each side of the third ventricle and axons from these neurons trace laterally and anteriorly to the location of the magnocellular neurons of the supraoptic nucleus just lateral to and above the optic chiasm. Axons of the supraoptic nucleus join axons of the paraventricular nucleus and course to the basal hypothalamus where they join the axons from the other side and course through the infundibular stalk to the axon terminals in the posterior pituitary.
The anatomic location of the physiologic regulators of osmotic and pressure/volume (baroreceptor) are located in vastly different sites. Osmoreceptors that control both thirst and osmotic regulation of vasopressin are located in the hypothalamus just anterior to the third ventricle, so the entire regulation of osmotic-induced changes in thirst and vasopressin secretion resides in a small and discrete area in the hypothalamus. Increases in osmolality stimulate the osmoreceptor to send a positive signal to stimulate thirst and to release vasopressin. For volume and pressure regulation, the receptors are located in the chest. There are high-pressure arterial baroreceptors in the carotid sinus and aortic arch and low-pressure volume receptors in the atria and pulmonary venous system. The pressure/volume receptor afferent signals are carried through cranial nerves 9 and 10 and synapse in the medulla before carrying their input to the magnocellular neurons. Both excitatory and inhibitory baroreceptor influences act on the magnocellular neurons, although some evidence indicates that a predominant mechanism involves the tonic inhibition of vasopressin secretion. A decrease in pressure/volume decreases the inhibition and stimulates release of vasopressin.
Vasopressin and oxytocin are nonapeptides that are synthesized in the cell bodies of the magnocellular neurons as part of a larger precursor peptide. Like other polypeptide hormones, the precursor proteins traverse the endoplasmic reticulum and the Golgi apparatus to be packaged in secretory granules. The neurosecretory granules then travel down the long axons through the stalk of the infundibulum to the posterior pituitary where the granules are stored. During transport, peptide enzymes (peptidases) cleave the prohormone into the hormone (vasopressin or oxytocin), a carrier protein (neurophysin), and (for vasopressin only) a glycopeptide. The synthesis of oxytocin and vasopressin is in separate neurons organized in clusters within the paraventricular nuclei and supraoptic nuclei. This allows stimulation of individual neurons and independent release of individual hormones. The stimulus for secretion of vasopressin or oxytocin is by neurotransmitters acting on the appropriate magnocellular cell body. An action potential propagates along the axon, causing an influx of calcium at the axon terminal and release of the hormone content of neurosecretory granules into the perivascular space.
Pathophysiology
All defined pathophysiology of the posterior pituitary is related to the function of vasopressin rather than oxytocin. Both decreased function of vasopressin and increased function of vasopressin manifest as abnormalities of water balance and depend upon action of vasopressin on the V2 receptor of the kidney rather than the V1 vascular receptors. The clinical presentation of low vasopressin function (diabetes insipidus) or excess vasopressin function (syndrome of inappropriate secretion of antidiuretic hormone, SIADH) is determined by the physiology of thirst. In humans thirst is well regulated to turn on when needed but not well regulated to turn off when not needed. When vasopressin is decreased or absent (diabetes insipidus), there is abnormal excretion of a large volume of dilute urine. This should cause hyperosmolality and an increase in serum sodium. However, as illustrated in Figure 5–2, an elevated plasma osmolality stimulates thirst, and a patient will drink a large volume of fluid to keep their sodium in the normal range. So, the clinical presentation of decreased vasopressin is one associated with polyuria and dilute urine together with polydipsia but with a normal serum sodium. Alternatively, when vasopressin is increased, there is abnormal concentration of urine and retention of water. The volume expansion by water dilutes serum sodium. If thirst were inhibited by hypo-osmolality as efficiently as thirst is stimulated by hyperosmolality, then the decrease in osmolality would produce a profound decrease in fluid intake, and a normal serum sodium would be maintained by the natural loss of fluid with perspiration, gastrointestinal fluid loss, and the urine output necessary to excrete ingested osmoles produced by food intake. Thirst, however, is not sufficiently inhibited, and the volume expansion not only dilutes serum sodium, but induces sodium excretion to reduce extracellular volume, which worsens the hyponatremia. So, the presentation of increased vasopressin is a chemical presentation of hyponatremia. There is concurrent concentrated urine with no change in thirst, modest volume expansion, hyponatremia, and natriuresis.
The other physiologic principles which determine some of the pathophysiology of abnormal secretion of vasopressin are: osmolality is regulated by changes in water balance, and volume is regulated by changes in sodium balance. When in conflict, volume will be preserved at the expense of osmolality. Thus, as described later, if dehydration is produced by diabetes insipidus, sodium will be retained to protect volume, even if it aggravates hypernatremia. With SIADH when sodium is diluted and extracellular volume is expanded, natriuresis is initiated to decrease extracellular volume in spite of the need to retain sodium to correct the hypo-osmolality.
Deficient Vasopressin: Diabetes Insipidus
Diabetes insipidus is literally the excretion of a large volume of urine (diabetes) that is hypotonic, dilute, and tasteless (insipid). Patients present with polyuria. Other causes of polyuria such as osmotic diuresis that occur in diabetes mellitus or intrinsic renal disease must be excluded. Frequent urination, without an increase in urine volume, suggests a urologic abnormality. Most adults will tolerate polyuria without complaint until it exceeds 3 to 4 L/d. If polyuria is shown to be dilute, the pathophysiologic mechanisms include: (1) primary ingestion of excess fluid, (2) abnormally decreased synthesis and secretion of vasopressin, (3) increased metabolism of vasopressin, and (4) decreased end-organ response to vasopressin.
Primary polydipsia with ingestion of large quantities of water produces modest decreases in plasma osmolality, decreased secretion of vasopressin, and excretion of profound quantities of urine. As the volume of dilute fluid delivered to the collecting duct is approximately 18 L/d, this quantity of dilute urine can be excreted on a daily basis while maintaining serum osmolality in the normal range. Virtually any pathologic process in the hypothalamus that causes diabetes insipidus may rarely cause primary stimulation of the thirst center and primary polydipsia on an organic basis. Alternatively, primary polydipsia may occur as a behavioral abnormality in psychiatric patients in whom the excess water ingestion and urination may represent delusions tied to bodily cleansing. The large volume of urine passing through the collecting duct in these patients washes urea out of the inner medulla and reduces the inner medulla osmolality. Additionally, the chronic suppression of vasopressin release and lack of action of vasopressin on the renal collecting duct decreases the synthesis of aquaporins in the collecting duct cells, adding to the inability to concentrate the urine maximally. Each of these abnormalities returns to normal in several days to weeks after fluid ingestion is decreased to normal.
Primary polyuria may be induced in a normal person if excess fluid is administered parenterally. This occasionally occurs in postoperative cases that do not involve the pituitary. In many surgical procedures, the stress of the surgery is a stimulus to release of vasopressin, and administered fluids may be retained during the procedure. Postoperatively, the stress is released, and patients have a normal diuresis of the retained fluids. If this normal excretion of fluid is matched by increased fluids administered parenterally, the patient will continue to excrete large volumes of urine. If the administered fluid is normal saline, the patients will excrete a large volume of isotonic urine. If the volume administered is sufficiently great, the patients will be unable to dilute or concentrate their urine above or below an iso-osmotic level regardless of therapeutic agents. The large osmotic load delivered to the kidney may make the patient unresponsive even to administered desmopressin, producing an iatrogenic therapeutic dilemma.
It is important to remember that the complaint of polyuria is based upon the volume of the urine not the urinary concentration. Therefore, as evident from Figure 5–2, considerable loss of ability to secrete vasopressin may occur before there is much loss of ability to concentrate the urine and even greater loss may occur before there is a noticeable increase in urine volume.
Familial diabetes insipidus may be caused by an autosomal dominant mutation in the vasopressin gene. Usually the mutation involves DNA sequences in the neurophysin or signal peptide region of the precursor gene rather than the region encoding vasopressin itself. These mutations cause abnormal folding of the precursor protein which produces abnormal trafficking and accumulation of a mutant prohormone in the endoplasmic reticulum. This produces alterations in packaging of the prohormone into neurosecretory granules in the Golgi apparatus. By ill-defined mechanisms, this leads to cell death of the vasopressin-producing neurons. Because the pathology in these neurons develops over time, young children may have normal urine output, and diabetes insipidus is not expressed until late childhood. An autosomal dominant form of diabetes insipidus also occurs in association with diabetes mellitus, optic atrophy, and deafness (DIDMOAD), also known as Wolfram syndrome.
The most common solid tumor to produce diabetes insipidus is craniopharyngioma. Suprasellar germinoma or pinealoma, which may be associated with precocious puberty, commonly produces diabetes insipidus. Metastatic disease to the pituitary hypothalamic area, for example, from breast or lung cancer, is more likely to produce diabetes insipidus than a deficiency of anterior pituitary hormones because the metastases lodge in the portal system of the hypothalamus where the vasopressin axons from the two sides join to form the pituitary stalk. Usually, there are widespread unidentified metastatic lesions elsewhere. Lymphoma or infiltration of the hypothalamus with leukemia are rare causes of diabetes insipidus.
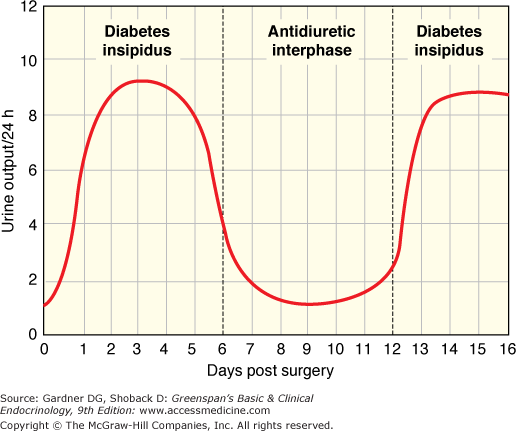
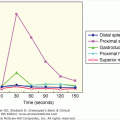
The anatomy of the neurohypophyseal system aids our understanding of this pathology. Hormone synthesis and regulation of release is high in the hypothalamus. Surgery or trauma at the level of the pituitary traumatizes only the axon terminals. Trauma of the axon terminals may disrupt the release of hormone, but usually this produces only a transient inability to secrete hormone and transient diabetes insipidus. Sectioning of the posterior pituitary stalk produces a characteristic pattern of diabetes insipidus known as the triphasic response. This is illustrated in Figure 5–3. Initial axonal shock inhibits the release of any vasopressin, and there is a period of diabetes insipidus that lasts for 5 to 10 days. Then, the severed axons in the posterior pituitary become necrotic and there is uncontrolled release of vasopressin. If excess fluid is administered during this time, the syndrome of inappropriate antidiuretic hormone is produced with hyponatremia (described later). This lasts for 5 to 10 days until all of the residual vasopressin leaks from the axon terminals when there is return of diabetes insipidus. While the classic response to stalk section is the three phases, in many clinical situations not all three phases occur. The most common occurrence after surgery in the pituitary or hypothalamus is transient diabetes insipidus that lasts a few days without subsequent sequelae. With partial damage to the pituitary stalk rather than total section, it is possible that the second phase will occur without preceding or subsequent diabetes insipidus (ie, isolated second phase). In the isolated second phase, there are sufficient normally functioning vasopressinergic neurons to avoid diabetes insipidus in the first and third phase; however, the damaged axon terminals leak vasopressin that will, when coupled with excess fluid intake, produce SIADH and hyponatremia. Characteristically, hyponatremia occurs 5 to 10 days after pituitary surgery, lasts for 5 to 10 days, and then resolves. Late occurrence of diabetes insipidus, that is, third phase without a first or second phase, is distinctly uncommon.
Figure 5–3
Illustration of the phases of urine output after section of the pituitary stalk. The triphasic response consists of: (1) diabetes insipidus due to axonal shock and lack of release of vasopressin; (2) an antidiuretic interphase when vasopressin leaks from the severed neurons; and (3) return of diabetes insipidus when the store of vasopressin in the posterior pituitary is depleted.
Patients with diabetes insipidus due to surgery or trauma may eventually recover. The level of section determines the number of magnocellular neurons that are actually killed. The higher in the stalk and the closer the section to the perikaria, the greater the number of neurons that die. Vasopressin-producing neurons whose axons terminate in the hypothalamus and serve as secretagogues for ACTH in the anterior pituitary may persist and hypertrophy after surgery or trauma. Additionally, branching of axons may develop in the hypothalamus after surgery or trauma, and these branches may generate new connections of vasopressinergic neurons to blood vessels. By these mechanisms, sufficient vasopressin function may return (usually within 1 year) to have normal fluid balance and no symptoms of diabetes insipidus.
Langerhans cell histiocytosis is the generic term that includes severe fulminant visceral Letterer-Siwe disease, multifocal Hand-Schuller-Christian disease, and benign eosinophilic granuloma. Diabetes insipidus is part of the central nervous system involvement in these disorders and is associated with other abnormalities of the head involving the cranial bones, oral mucosa, or brain. Systemic manifestations in the lung, bone, and skin may also be present. Wegener granulomatosis and sarcoidosis may also have diabetes insipidus as a part of the central nervous system pathology.
It is now recognized that many cases of diabetes insipidus, that were previously termed idiopathic and for which there was no specific etiology, are probably due to lymphocytic infiltration of the neurohypophysis on an autoimmune basis. X-rays may reveal enlargement of the pituitary stalk as evidence of the lymphocytic invasion. There may be resolution of the autoimmune response with time and the stalk may return to normal size, but the diabetes insipidus is usually permanent.
A rare variant of diabetes insipidus involves an absent osmoreceptor function, but an intact baroreceptor function. In this form of diabetes insipidus, patients do not sense thirst and, therefore, do not drink water. This results in an elevation of serum osmolality because the osmostat does not respond to the increased osmolality by releasing vasopressin or stimulating thirst. However, vasopressin synthesis and storage are normal as demonstrated by specific tests of baroreceptor function. Characteristically, these patients do not drink water and become sufficiently dehydrated that their baroreceptors stimulate vasopressin release, they then remain in a balanced situation with an elevated serum sodium level, relatively concentrated urine, and lack of thirst.
Brain dead patients who are on life support often have diabetes insipidus as part of the central nervous system pathology. It is standard practice to maintain electrolyte and water balance for maximal preservation of organs for transplantation.